Validation Requirements for Pharmaceuticals

Why take this course?
Course Title: Validation Requirements for Pharmaceuticals
Headline: Master the Practical Approach for Validation Requirements as Per FDA, EU, and More with Expert Instructor Hitendrakumar Shah!
Course Description: Dive deep into the complex world of pharmaceutical validation with our comprehensive online course. This meticulously crafted curriculum is designed to provide you with a thorough understanding of stability study requirements, process validation, analytical method validation, water system validation, and equipment qualification, all grounded in the latest FDA, EU, PIC/S, and other regulatory guidelines.
Course Overview:
- Introduction: Begin your journey by gaining an initial understanding of the critical concepts of validation and qualification within the pharmaceutical industry.
Key Learning Areas:
🔍 Qualification Learning:
- Concepts: Delve into the principles of Validation & Qualification, learn how to effectively organize and plan for validation activities, and understand the intricacies of VMP (Validation Master Plan), VP (Validation Procedure), VR (Validation Reports), and VMR (Validation Master Record).
- Quality Risk Management (QRM): Explore the role of QRM in enhancing the qualification process.
- Process Flow: Navigate through the steps involved in a comprehensive qualification process, including analytical instrument qualification according to USP standards.
🚀 Process Validation Learning:
- Guideline Expectations: Get up to speed with the expectations of various regulatory bodies such as EU, FDA, ANVISA, TGA, WHO, and more.
- Regulatory Expectations: Understand both traditional and hybrid approaches to process validation, grasp the importance of Continued Process Verification (CPV) versus Product Quality Review (PQR), and learn how to design a robust process validation protocol and report.
- Comparative Analysis: Gain insights into the detailed differences between EU and FDA guidelines for process validation.
🧪 Analytical Method Validation Learning:
- FDA Expectations: Comprehend the FDA's expectations for analytical method validation, including a brief on the QBD approach and the role of QRM.
- ICHQ14 & ICHQ2(R1): Deep dive into ICHQ14 - Analytical Method Development and ICHQ2(R1) – Analytical Method Validation, covering all validation parameters and the overall evaluation process.
- Revalidation: Learn about revalidation criteria and how to effectively approach method validation practically.
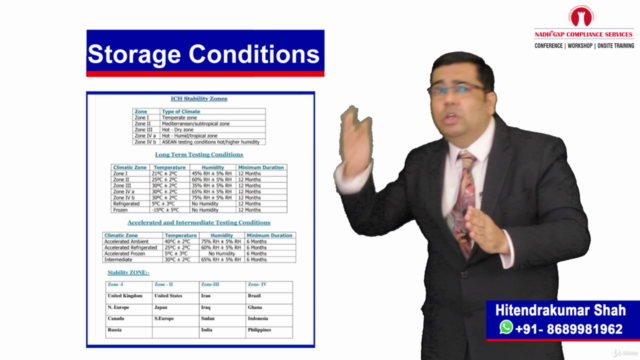
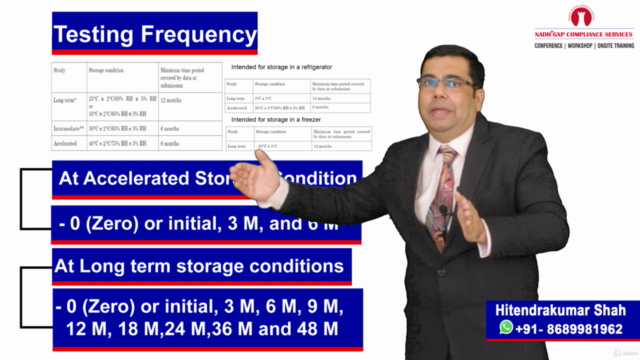
💧 Water System Validation Session:
- Comprehensive Coverage: Grasp the importance of water in pharmaceuticals, understand the qualification and validation of water purification systems, and identify the suitability of water systems.
- Life Cycle Approach: Explore the three-phase approach to Process Qualification (PQ) or water system validation, tackle sampling issues and common problems, and develop a comprehensive water system validation strategy.
- Regulatory Insights: Discuss FDA warning letter observations and ensure your water systems meet all necessary standards.
Why Enroll?
- Expert Led: Learn from the best with industry expert Hitendrakumar Shah guiding you through every concept.
- Practical Application: Gain hands-on knowledge that can be directly applied to real-world scenarios in your field.
- Regulatory Mastery: Master the nuances of different regulatory guidelines and ensure compliance with global standards.
- Quality Education: Benefit from a curriculum that is designed to update and refine your skills and knowledge in pharmaceutical qualification and validation.
Enroll today and take a significant step towards becoming an authority on validation requirements for pharmaceuticals! 🚀💊🌍
Course Gallery


Loading charts...