Quality Risk Management in Pharmaceuticals ICHQ9(R1)

Why take this course?
Course Title: Mastering Quality Risk Management in Pharmaceuticals - ICH Q9(R1)
Headline: Navigate the Complexities of Pharma Quality Risk with Confidence! 💊✨
Course Description:
Dive into the world of Quality Risk Management (QRM) with our comprehensive online course, designed to equip you with a deep understanding of the ICH Q9(R1) guideline, the cornerstone for risk management in pharmaceutical quality. This course is meticulously crafted for professionals across the pharmaceutical industry, including quality assurance and control specialists, regulatory affairs managers, and anyone looking to master the intricacies of QRM.
Key Learnings:
-
Introduction to Quality Risk Management (QRM): Gain a foundational understanding of QRM and its significance in ensuring product quality and patient safety.
-
ICH Quality Guidelines Overview: Familiarize yourself with the International Council for Harmonisation (ICH) guidelines, and understand their role in the pharmaceutical industry.
-
Updates in ICH Q9(R1): Discover the latest updates in the revised ICH Q9(R1) guideline and how they impact your quality management processes.
-
Integration of ICH Q9 with ICH Q8 and ICH Q10: Learn how ICH Q9 complements other guidelines like Q8 and Q10 to enhance the overall quality of pharmaceutical products.
-
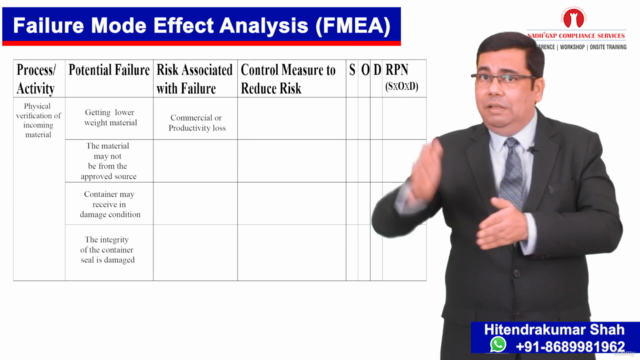
Practical QRM Process: Engage with hands-on learning on performing a QRM process using tools such as Failure Mode Effect Analysis (FMEA), Action Priority Tables, and Heat Map Matrix.
-
Vote of Thanks - Q&A Session: Interact with the instructor during a live Q&A session to clarify your doubts and express your views on the topic.
Course Highlights:
-
📚 Comprehensive Video Lectures: Our expert course instructor, Hitendrakumar Shah, delivers insightful video lectures that simplify QRM principles and their practical implementation.
-
🔍 Real-World Examples: Learn through practical examples, making complex concepts easy to understand and apply in real-world scenarios.
-
🚀 Good Review Practices: Understand the principles of good review practices with a focus on compliance, including examples from FDA citations and regulatory expectations.
-
🛠️ Actionable Tools: Explore action priority tables, heat maps, and other tools that will help you manage risks effectively.
-
🎓 Qualify Reviewers: Learn how to identify and qualify reviewers to ensure a robust quality system.
Course Format:
- Engaging video lectures
- Interactive Q&A sessions
- Real-world examples and case studies
- Quizzes and practical exercises
- Accessible learning materials
Who Should Attend?
This course is ideal for:
- Quality Assurance (QA) professionals
- Quality Control (QC) specialists
- Regulatory Affairs managers
- Pharmaceutical scientists
- Any individual interested in pharmaceutical quality and risk management
Embark on your journey to excellence in Quality Risk Management within the pharmaceutical industry. With our expert-led course, you'll not only understand the critical role of ICH Q9(R1) but also learn how to implement these principles effectively to ensure product quality and patient safety. Enroll today and transform your approach to risk management! 🎓💡
Course Gallery



Loading charts...