Quality in Pharmaceutical Industry: GMP & Process Validation

Why take this course?
🚀 Welcome to Mastering Quality in the Pharmaceutical Industry! 💊
Are you ready to dive deep into the world of Good Manufacturing Practice (GMP) and Process Validation? If so, this course is your golden ticket to understanding the critical aspects of quality management within the pharmaceutical industry. Taught by the esteemed Aydan Ozdenc, this comprehensive training will cover everything from the basics to the intricate details that ensure your products meet stringent regulatory standards set by the FDA, EMA, and more.
🔥 Course Title: Quality in Pharmaceutical Industry: GMP & Process Validation
📘 What You'll Learn:
1. INTRODUCTION 🌍
- Understand the importance of quality in pharmaceuticals and the role of GMP & Process Validation.
2. GENERAL DEFINITIONS FOR PROCESS VALIDATION 📚
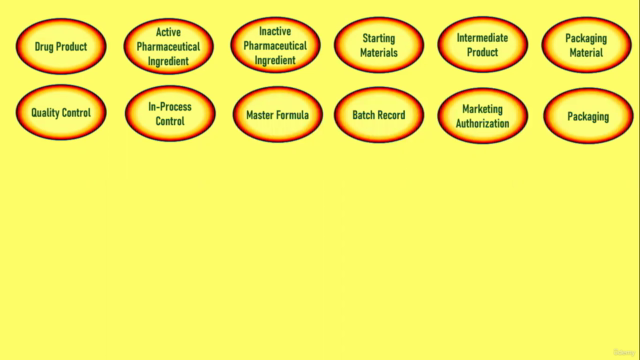
- Learn about the various components that define process validation, from drug products to manufacturing flow charts (👉 Full List Here).
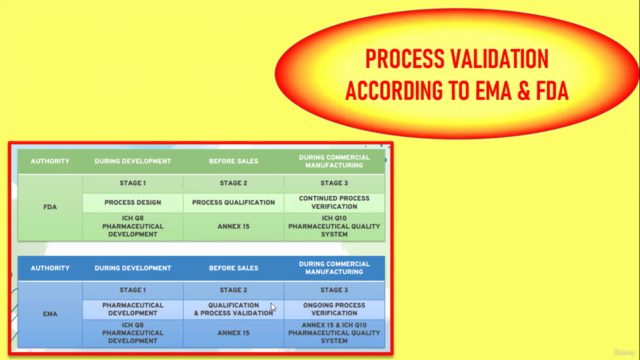
3. PROCESS VALIDATION ACCORDING TO EMA & FDA 🏫
- Get insight into how different regulatory bodies approach process validation and the key elements they focus on.
4. INTRODUCTION TO PROCESS VALIDATION 🎓
- Discover the principles and aims of process validation, as well as the best strategies to effectively implement them in your processes.
5. GENERAL CONSiderations FOR PROCESS VALIDATION 🤔
- Explore the overarching considerations that are crucial for successful process validation.
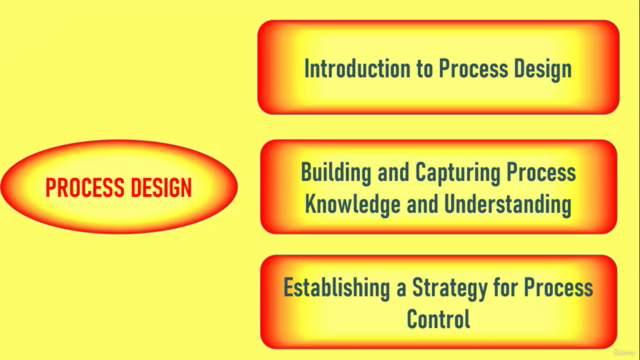
6. PROCESS DESIGN 🛠️
- Dive into the design phase, where you'll learn how to build and capture knowledge about your process to lay a solid foundation for control and validation.
7. PROCESS QUALIFICATION 🔬
- Understand the steps involved in qualifying your process, including designing facilities, qualifying utilities and equipment, and conducting process performance qualification.
8. CONTINUED PROCESS VERIFICATION 🔄
- Learn about the ongoing monitoring techniques that ensure processes continue to perform as intended.
9. CONCLUSION ✍️
- Wrap up your learning journey with a solid understanding of how to maintain quality throughout the pharmaceutical lifecycle.
🚀 Course Highlights:
- Expert-Led Learning: Benefit from Aydan Ozdenc's years of experience in the field.
- Comprehensive Coverage: From general definitions and FDA/EMA standards to process design, qualification, and continued verification.
- Practical Insights: Real-world examples and case studies that bring theoretical knowledge to life.
- Actionable Knowledge: Practical tools and methodologies you can apply immediately to enhance your processes.
🎓 Who Is This Course For?
- Quality Assurance & Control Professionals
- Regulatory Affairs Specialists
- Biotech and Pharma R&D Scientists
- Manufacturing Personnel
- Students in Life Sciences or related fields
👩🏫 Educator Profile: Aydan Ozdenc is a seasoned expert with extensive knowledge and hands-on experience in GMP & Process Validation. With a career spanning across various roles within the pharmaceutical industry, Aydan brings a wealth of practical insights that will be invaluable to your professional development.
📅 Enroll Now! Take the first step towards excellence in pharmaceutical quality management. Enroll in this course and secure your spot among the quality champions of the industry. 🏅
Ready to elevate your understanding of GMP & Process Validation? Let's embark on this journey together and achieve mastery in ensuring the highest quality standards for life-saving pharmaceutical products! 🚀✨
Course Gallery

Loading charts...