Quality in Pharma Industry : Analytical Method Validation

Why take this course?
🧬 Quality in Pharma Industry: Analytical Method Validation 🚀
Are you ready to dive into the world of pharmaceutical quality assurance and control? Our comprehensive online course, led by industry expert Aydan Ozdenc, is designed to give you a deep understanding of analytical method validation within the pharma industry. This is not just about following protocols; it's about mastering the science behind ensuring that every drug product meets the highest standards of quality for patient safety and efficacy.
Course Overview 📚
Why This Course? Analytical methods are pivotal in determining the quality, composition, and purity of active pharmaceutical ingredients (APIs) and finished drug products. These methods are a cornerstone for marketing applications and are crucial for product lifecycle management. Global guidelines published by the International Conference on Harmonization (ICH) and the Food and Drug Administration (FDA) set the benchmarks for method development and validation, ensuring consistency and quality across different regions and regulatory environments.
Course Highlights ✨
- Comprehensive Understanding: Learn about the full spectrum of analytical test methods used in the pharmaceutical industry, from their introduction to their practical application.
- Drug Substances & Products Analysis: Gain expertise in the various types of analytical tests for drug substances and products, including identification, assay, content uniformity, and impurity analysis.
- Regulatory Guidance: Explore the scope of ICH guidelines regarding analytical test methods and understand their role in ensuring method validation meets regulatory standards.
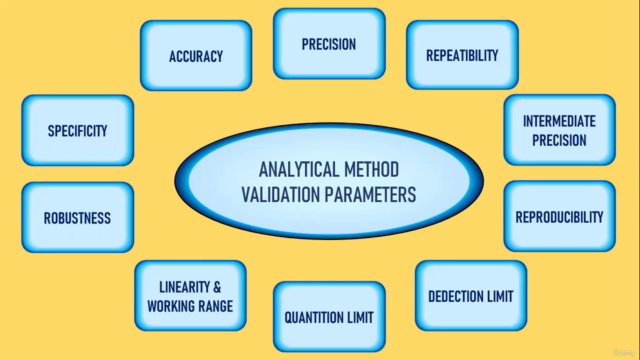
- Validation Parameters: Master the key parameters of analytical method validation, such as specificity, accuracy, precision (including repeatability, intermediate precision, and reproducibility), detection and quantitation limits, linearity, range, and robustness.
- Step-by-Step Validation Process: Follow a clear, step-by-step guide to the analytical method validation process, ensuring you can apply these principles in real-world scenarios.
Course Content Breakdown 📊
-
Introduction
- Overview of the importance of quality in the pharma industry.
-
Analytical Test Methods in Pharmaceutical Industry
2_1. Introduction to Analytical Test Methods 2_2. Categories of Analytical Test Methods
-
Analytical Test Methods for Drug Substances
3_1. Description of the process 3_2. Detailed exploration of identification, assay, content uniformity, and impurity analysis.
-
Analytical Test Methods for Drug Products
4_1. Description of the process 4_2. Identification, assay & content uniformity, impurities analysis for drug products.
-
ICH Guideline Scope for Analytical Test Method
- Understanding the ICH guidelines and their implications.
-
Introduction to Analytical Method Validation
6_1. Types of analytical methods that require validation. 6_2. In-depth look at the parameters of analytical method validation. 6_3. Definitions and expectations for a validated method.
-
Analytical Method Validation Parameters
7_1. Specificity 7_2. Accuracy 7_3-7_8. Precision (including repeatability, intermediate precision, reproducibility), detection and quantitation limits, linearity, range, and robustness.
-
Analytical Method Validation Steps
- A detailed walkthrough of the validation process from start to finish.
-
Conclusion
- Summarize the key takeaways and how they apply to your role in ensuring drug quality.
Who Should Take This Course? ✈️
This course is ideal for:
- Quality Assurance (QA) and Quality Control (QC) professionals who are new to the pharma industry or looking to expand their knowledge.
- Regulatory Affairs experts seeking a deeper understanding of method validation.
- Analytical scientists and technicians involved in drug analysis, formulation, and product development.
- Students and academic researchers interested in the pharmaceutical industry's quality control and validation processes.
Embark on this journey with Aydan Ozdenc and elevate your expertise in pharmaceutical quality assurance and control. Enroll now and unlock the door to a career where you can ensure the safety and efficacy of life-changing medications! 💊💪
Enroll Now to secure your spot in this transformative learning experience!
Course Gallery



Loading charts...