Validation of PLC

Why take this course?
🎓 [Course Title] Validation of PLC
[Course Headline] A brief understanding and compliance to PLC Validation for Pharmaceuticals
About the Course:
Embark on a comprehensive journey into the realm of Process Logic Controllers (PLC) in the pharmaceutical industry with our specialized online course. Designed by industry expert Hitendrakumar Shah, this course is tailored for professionals looking to master the intricacies of PLC validation, ensuring compliance and quality assurance within cGXP frameworks.
Why Take This Course?
- Understand Common Errors: Gain insights into the frequent pitfalls associated with cGXP computerized systems and how to avoid them.
- Expert-Led Lectures: Access a series of recorded lectures that provide an in-depth understanding of HMI, PLC, SCADA, and their role in pharmaceutical validation.
- Validation Mastery: Learn the critical components of computerized system validation, from basics to advanced strategies, including the latest updates from GAMP 5.
- Real-World Applications: Apply your knowledge with practical examples and real-world case studies that reflect industry standards.
Course Structure:
1️⃫ Introduction to HMI, PLC & SCADA:
- Basics of HMI, its industrial usage, and benefits.
- A comprehensive understanding of PLC, major considerations in the Unified Requirements Specification (URS), and discussion on the drawbacks of PLC.
- Insights into Brief Understanding of SCADA, Components of SCADA, and their integration within pharmaceutical environments.
2️⃫ Computerized System Validation Basics:
- The importance of computerized system validation in Pharma 4.0.
- Detailed coverage of GAMP5 guidelines, hardware and software categorization, validation strategies, and the full spectrum of validation activities from IQ to PQ.
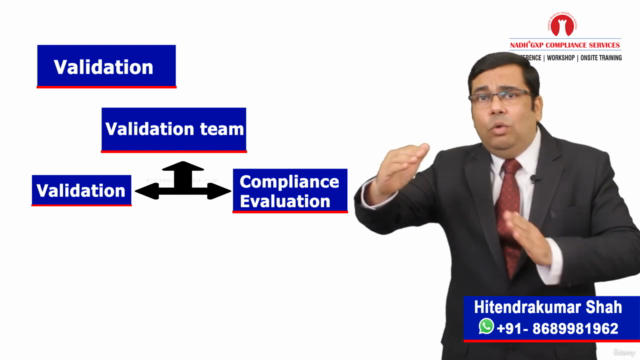
- Understanding the difference between a Validation Plan and a Validation Master Plan, and the role of Risk Assessments in the validation process.
3️⃫ PLC Validation Strategy:
- A primer on the concept of PLC validation, requirements for GXP computerized systems, and the validation of PLC logic.
- A comprehensive overview of Traceability Matrix, Functional Risk Assessment (FRA), Infrastructure Risk Assessment, and Process Control System validation requirements.
- Identification of critical GXP and non-GXP systems, system complexity, and the validation of PLC logic.
4️⃫ PLC Validation (Session) II:
- A quick recap of Session – 1 with an emphasis on different test strategies and white box vs. black box testing.
- Exploring the Agile testing approach in the context of PLC validation, its difference from the Waterfall approach, and the nuances of Verification vs. Validation.
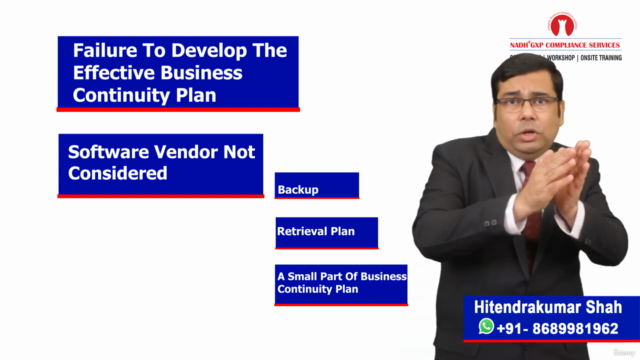
- Practical insights into QTP (Quick Test Professional) for automated testing, stages of Manual testing, and preparing for a System Retirement Plan.
Learning Outcomes:
- Comprehensive Understanding: Gain a thorough understanding of PLC validation within the pharmaceutical industry.
- Validation Mastery: Master the techniques and strategies required to validate PLC systems in compliance with cGXP guidelines.
- Risk Assessment: Learn to conduct effective Risk Assessments to ensure system integrity.
- Testing Techniques: Explore various testing methodologies, including Agile approaches, to enhance your validation process.
- Future-Ready Skills: Equip yourself with the knowledge to adapt to future industry changes and updates in regulatory guidelines.
Who Should Take This Course?
This course is designed for:
- Engineers and Technicians working with PLCs in pharmaceutical settings.
- Quality Assurance and Quality Control Professionals.
- Validation Specialists transitioning into or within the pharmaceutical industry.
- Students and academics interested in the intersection of technology and pharmaceuticals.
Join us on this educational quest to master PLC validation, ensuring your pharmaceutical operations are not only effective but also compliant with international standards. 🧫⚙️✅
Course Gallery


Loading charts...