Physical Chemistry - Structure of Atom

Why take this course?
🌟 Complete Chemistry for Engg and Medical Entrance Exam Preparation (IIT JEE Main | Advanced | BITSAT | SAT | NEET, etc.) 🌟
SUMMARY:
-
Atomic Basics: Dive into the fascinating world of atoms, the fundamental units of matter. John Dalton's atomic theory laid the foundation for our understanding of chemical reactions at the atomic level. But as science evolved, we discovered that atoms are not indivisible; they are composed of even smaller particles: electrons, protons, and neutrons.
-
Historical Models: Journey through the history of atomic models with William Thomson's plum pudding model, which depicted atoms as positive spheres with embedded electrons. However, this model was disproven by Hans Geiger and Ernest Marsden's famous gold foil experiment conducted by Ernest Rutherford in 1909, leading to the solar system-like model where a tiny, positively charged nucleus houses orbiting electrons.
-
Rutherford's Model vs. Stability: While Rutherford's model was an improvement over Thomson's, it failed to explain why electrons don't fall into the nucleus and the orderly arrangement of electrons around the nucleus.
-
Bohr's Revolutionary Breakthrough: Fast forward to 1913 when Niels Bohr introduced his model for the hydrogen atom, solving the stability issue by proposing that electrons move in circular orbits at specific energy levels. His model, while accurate for hydrogen, could not explain complex atoms with multiple electrons due to the limitations imposed by quantum mechanics.
-
Quantum Leap: Erwin Schrödinger's groundbreaking Schrödinger equation of 1926, incorporating Louis de Broglie's wave-particle duality, provided a comprehensive solution that explained electron distributions and allowed energy levels in atoms. The equation naturally led to the concept of quantum numbers (n, l, ml) that define the structure of atoms at a quantum level.
-
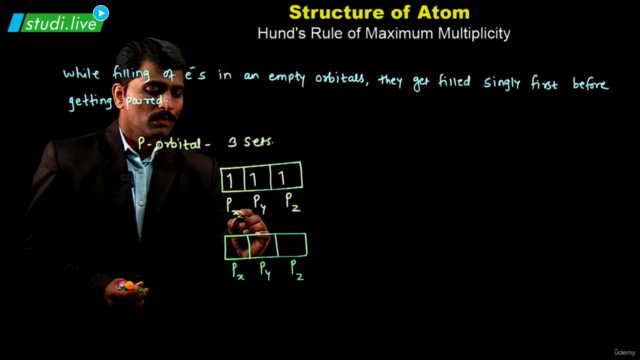
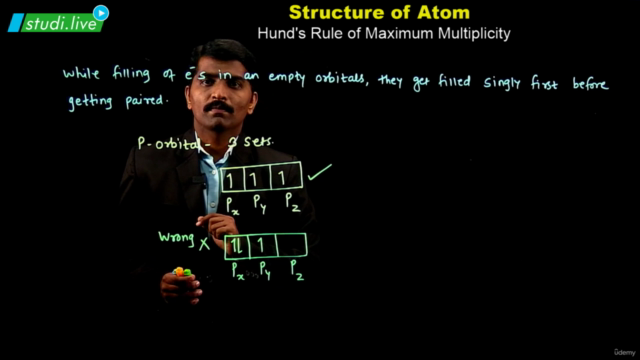
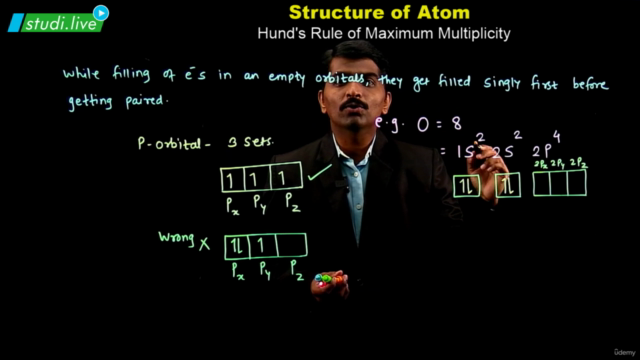
Electron Configuration: Understanding the electronic structure of an atom is crucial. Atoms with multiple electrons have complex structures with shells, subshells, and orbitals. The energy of these orbitals depends on the values of n and l, with lower (n + l) values indicating lower energy states. The Pauli exclusion principle and Hund's rule guide the filling of electrons in these orbitals.
Why Study This?
-
Essential for Entrance Exams: A deep comprehension of atomic structure, as described by quantum mechanics, is critical for acing engineering and medical entrance exams like IIT JEE Main, Advanced, BITSAT, SAT, and NEET.
-
Foundation for Chemistry: This knowledge forms the foundation for understanding chemistry, which is essential not only in the fields of engineering and medicine but also in materials science, physics, and beyond.
-
Quantum Mechanics Mastery: By mastering the principles of quantum mechanics and atomic structure, you'll gain a profound understanding that will serve as a key to solving complex problems and preparing for competitive exams.
Key Takeaways:
-
Historical Context: Learn about the evolution of atomic models from Dalton to Rutherford to Bohr and finally to quantum mechanics.
-
Quantum Numbers: Grasp the significance of quantum numbers (n, l, ml) in defining an atom's electron configuration.
-
Energy Levels: Understand how the energy levels of electrons are determined by their position within the atom, as defined by the Schrödinger equation.
-
Pauli Exclusion and Hund's Rule: Know when to apply these principles in the context of electron configuration.
🎓 Ready to Master Atomic Structure? 🎓
Join our comprehensive course designed specifically for engineering and medical entrance exam preparation. With expert instructors and interactive sessions, you'll not only understand the intricacies of atomic structure but also excel in your chosen field. Get ready to unlock the secrets of the universe and ace your exams with confidence! 🌈
Course Gallery

Loading charts...