Physical Chemistry - Solid State

Why take this course?
🌟 Complete Chemistry for Engg and Medical Entrance Exam Preparation: Physical Chemistry - Solid State 🌟
SUMMARY
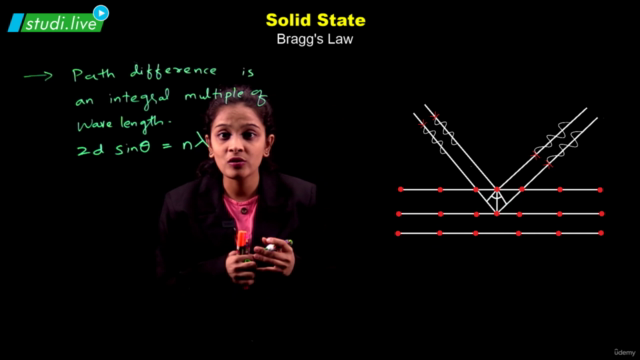
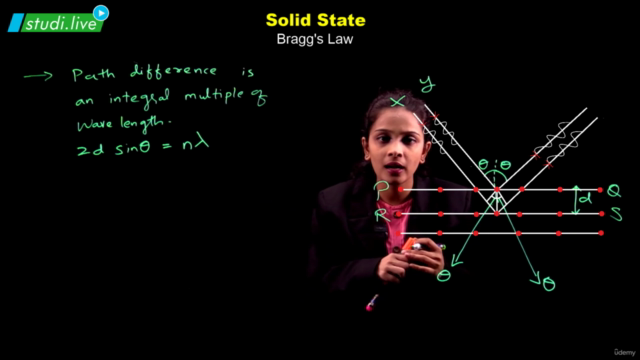
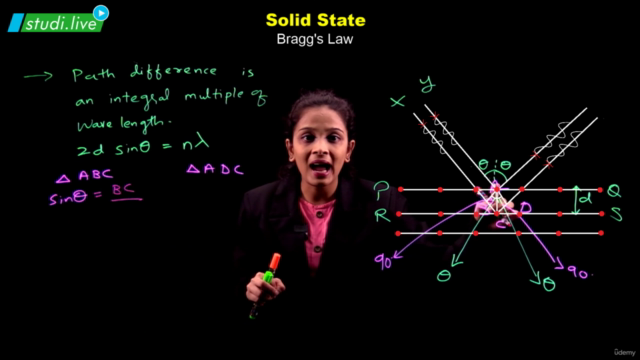
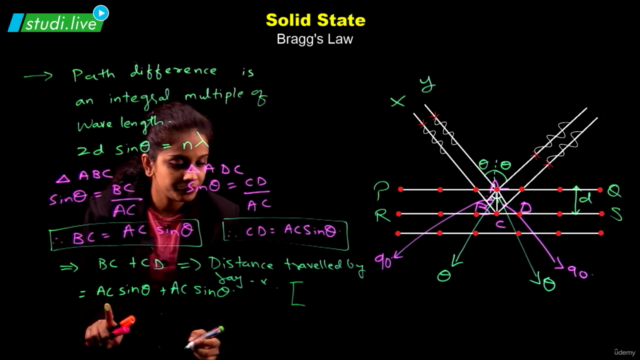
Solids, with their definite mass, volume, and shape, are composed of constituent particles arranged in a fixed position, exhibiting short distances and strong interactions. Amorphous solids possess only short-range order, resembling supercooled liquids without distinct melting points and showing isotropic behavior. In contrast, crystalline solids boast long-range order, anisotropy, and characteristic shapes; their particles interact in ways that define four primary categories: molecular, ionic, metallic, and covalent solids, each with distinct properties.
The crystalline solid structure is underpinned by crystal lattices, the most fundamental of which are the Bravais lattices. These can be categorized into 14 types based on different arrangements of particles. The basic unit of a crystal structure, known as the unit cell, defines the crystal's dimensions and angles between edges. Unit cells come in both primitive and centred forms, leading to a total of 14 Bravais lattices.
Among the efficient packing structures are the hexagonal close-packed (hcp) and cubic close-packed (ccp) or face-centred cubic (fcc) lattices, both of which occupy about 74% space and feature characteristic voids like octahedral and tetrahedral spaces. Less efficient packings include the body-centred cubic (bcc) lattice, with 68% space occupation, and the simple cubic lattice, with merely 52.4%.
The perfection of a crystal's structure is seldom absolute; it often contains various imperfections or defects, which are crucial in determining physical properties. These defects include point defects (stoichiometric, impurity, and non-stoichiometric) and line defects. In ionic solids, common point defects are Frenkel and Schottky defects. Impurities can create vacancies in the host lattice, leading to altered properties, especially in semiconductors used in the electronics industry.
Solids exhibit a range of magnetic properties, such as paramagnetism, diamagnetism, ferromagnetism, antiferromagnetism, and ferrimagnetism, which are pivotal in technologies like audio and video recording devices.
Key Takeaways:
- Solid State: Understanding the fundamental nature of solids, their order, and the interactions between particles.
- Crystal Lattices: Exploring the 14 Bravais lattices, including their unit cells and the concept of packing efficiency.
- Point and Line Defects: Identifying the different types of defects, their impact on crystal properties, and their applications in semiconductors.
- Magnetic Properties: Learning about how the electronic configuration of solids gives rise to various magnetic behaviors used in modern technologies.
Why Study Solid State Chemistry?
Mastering the concepts of solid state chemistry is essential for:
- Aspiring engineers and medical professionals who wish to excel in competitive exams like IIT JEE Main, Advanced, BITSAT, SAT, NEET, and more.
- Scientists and researchers who are interested in understanding material science and its applications in advanced technologies.
- Students aiming to explore the world of quantum mechanics and molecular orbital theory in solids.
What You Will Learn:
- The principles of solid state structures, including crystal lattices and unit cells.
- The types of solid states and their properties, with a focus on crystalline solids.
- The impact of point and line defects on the properties of materials.
- The significance of magnetic properties in various applications.
Join our online course "Physical Chemistry - Solid State" to delve into the intricate world of solid state chemistry, enhance your understanding, and prepare effectively for engineering and medical entrance examinations. Let's unlock the secrets of solids together! 🎓✨
Course Gallery
Loading charts...