Physical Chemistry - Ionic Equilibria

Why take this course?
🚀 Course Headline:
Unlock Your Potential: Master Physical Chemistry for Top Engineering & Medical Entrance Exams 🎓 (IIT JEE Main | Advanced | BITSAT | SAT | NEET & More!)
🎉 What You'll Learn:
Physical Chemistry - Ionic Equilibria: A Comprehensive Guide
Summary:
🧪 Introduction to Electrolytes and Solutions: Understand the world of electrolytes, including acids, bases, and salts, and their behavior in aqueous solutions. Dive into the concepts of ionization, dissociation, and conduction of electricity by learning about strong and weak electrolytes.
- Arrhenius Theory: Discover how acids release hydrogen ions and bases release hydroxide ions in solution.
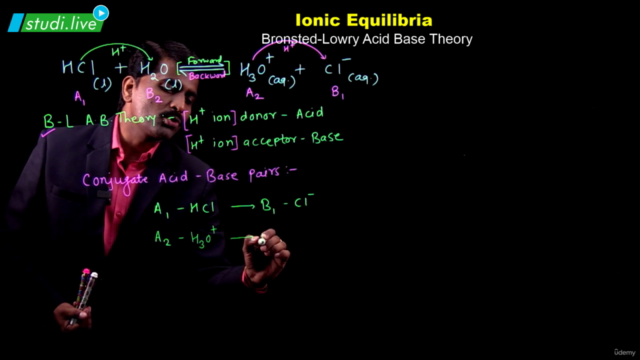
- Brönsted-Lowry Acid/Base Concept: Explore the dynamic relationship between acids, bases, and their conjugate pairs.
- Lewis Theory: Expand your understanding of acids and bases as substances that either donate or accept electron pairs.
📈 Ionization Constants and pH Scale: Master the calculations of ionization constants (Ka, Kb) and the importance of the pH scale in measuring hydrogen ion concentrations. Learn about the significant relationship between pH, pOH, pKw, and how it affects chemical reactions.
- Buffer Solutions: Understand the role of buffer solutions in controlling pH levels and their importance in biological systems and industrial applications.
🧵 Solubility Equilibrium and Common Ion Effect: Explore the solubility equilibrium of sparingly soluble salts and how the common ion effect influences their solubility. Learn to predict whether a salt will dissolve or precipitate under given conditions.
Suggested Activities for Students:
🔬 Practical Exercises:
- Use pH paper to determine the pH of various substances and solutions, including fruits, vegetables, soft drinks, body fluids, and water samples.
- Determine if salt solutions are formed from strong or weak acids and bases by observing their pH.
- Prepare buffer solutions using sodium acetate and acetic acid, then measure their pH.
- Explore the colors of different indicators in solutions with varying pH levels.
- Perform acid-base titrations to observe the behavior of indicators and neutralization points.
- Investigate the common ion effect on the solubility of sparingly soluble salts.
- Compare the results from pH paper with those obtained using a pH meter (if available).
📆 Course Outline:
- Electrolytes and Solutions
- Types of electrolytes, ionization, and dissociation
- Arrhenius Theory
- Acids, bases, and their ions in solution
- Brönsted-Lowry Acid/Base Concept
- Conjugate acid-base pairs and their equilibria
- Lewis Theory
- Electron pair theory of acids and bases
- Ionization Constants
- Ka, Kb, and their significance in weak electrolytes
- pH Scale and Ionic Equilibria
- Measuring and applying pH values, ionic equilibria, and the importance of pKa, pKb, and pKw
- Buffer Solutions
- Their composition and significance in controlling pH
- Solubility Equilibrium
- Ksp, sparingly soluble salts, and the influence of common ions on solubility
- Common Ion Effect and Precipitation
- Solubility rules and their exceptions
🎓 Why This Course?
This course is meticulously designed to enhance your conceptual understanding of physical chemistry, which is crucial for success in competitive exams like IIT JEE Main, Advanced, BITSAT, SAT, and NEET. By combining theory with practical activities, you'll gain a deep insight into the principles that govern chemical reactions and their environmental, biological, and industrial implications.
🕒 Who Should Take This Course?
- Aspiring engineers and medical professionals preparing for entrance exams
- Students who want to solidify their understanding of physical chemistry fundamentals
- Anyone interested in deepening their knowledge of chemical equilibria and reactions
🔥 Get Ready to Master Physical Chemistry! 📚 Enroll now and take the first step towards acing your entrance exams with confidence and clarity.
Course Gallery



Loading charts...