Preparation of Pharmaceutical Development Part for CTD

Why take this course?
🚑 Embark on a Journey into Pharmaceutical Innovation with Our Comprehensive Online Course!
Introduction to Pharmaceutical Development
Pharmaceutical development is the cornerstone of producing high-quality, reliable pharmaceutical products. It encompasses the design and optimization of a product's formulation and manufacturing process to consistently deliver the intended therapeutic performance. This course is your gateway into understanding the intricate process behind creating pharmaceuticals that meet the highest standards of safety and efficacy.
Course Overview
Our online course, meticulously crafted by industry expert Aydin Alpan, will guide you through the various aspects of pharmaceutical development, including:
-
Pharmaceutical Development Lifecycle
- Understanding the lifecycle from concept to commercialization.
-
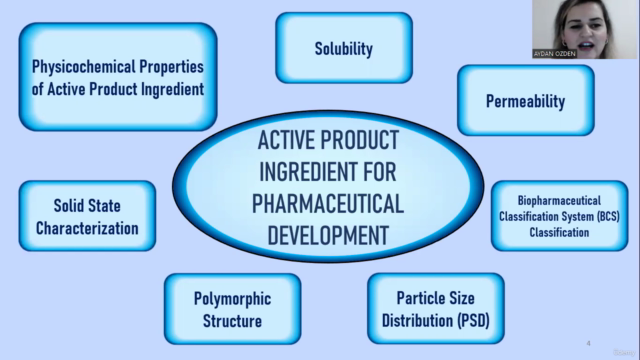
Physicochemical Properties
- Exploring the properties that influence drug design and stability.
-
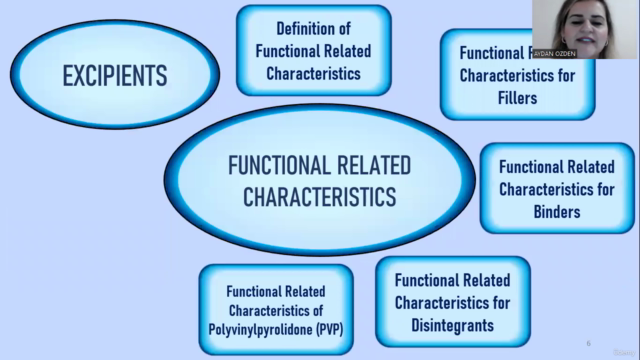
Excipient Selection & Role in Formulation
- Discovering the critical role of excipients and how they impact product performance.
-
Functional Related Characteristics (FRCs)
- Learning about FRCs and their significance in the development process.
-
Drug Product Design
- Diving into the principles of creating effective drug products.
-
Dissolution & Solubility Studies
- Mastering the science behind dissolution testing and its importance in drug development.
-
Stability and Degradation Studies
- Gaining insights into how drugs degrade and ensuring product stability.
-
Manufacturing Method Development
- Understanding the selection and development of manufacturing methods to scale up production.
Course Modules
🧬 Module 1: Physicochemical Properties & Solid-State Characterization
- Explore the impact of particle size, polymorphism, and solid-state form on drug performance.
🔬 Module 2: Degradation Studies & Stability Testing
- Learn about the importance of stability testing, degradation pathways, and how to design experiments to ensure product longevity.
💊 Module 3: Excipient Selection & Functional Related Characteristics
- Dive into the role of excipients in drug formulation and how to evaluate their impact on the final product.
🔄 Module 4: Drug Product Design & Development
- Discover the principles of immediate-release and extended-release formulations, and how to develop them for large-scale manufacturing.
🧪 Module 5: Dissolution Testing & Specifications
- Understand the role of dissolution in ensuring drug bioavailability and setting appropriate specifications.
🏫 Module 6: Manufacturing Method Development
- Learn about the critical steps in method selection, development, and validation for pharmaceutical products.
Learning Outcomes
By the end of this course, you will have a comprehensive understanding of the entire pharmaceutical development process. You'll be able to:
- Design and optimize drug formulations.
- Conduct and interpret stability and degradation studies.
- Select appropriate excipients and evaluate their functional related characteristics.
- Develop and validate manufacturing methods for consistent production.
- Ensure compliance with regulatory requirements and guidelines.
Who Should Take This Course?
This course is ideal for:
- Aspiring pharmaceutical scientists and professionals.
- Current industry practitioners looking to enhance their knowledge.
- Regulatory affairs specialists seeking a deeper understanding of the development process.
- Academics and students with an interest in advancing their research or studies in pharmaceutical science.
Enroll Now!
Embark on your journey to becoming a pharmaceutical expert today! 🌟 With our interactive course materials, real-world examples, and expert guidance, you'll be equipped to make significant contributions to the pharmaceutical industry.
Join us, and let's transform your passion for science into groundbreaking pharmaceutical advancements. Enroll in our Pharmaceutical Development online course now and unlock your potential! 🎓💪
Course Gallery


Loading charts...