Pharma Product Design, Technology & Commercial Manufacturing

Why take this course?
TDM Pharma Product Design, Technology & Commercial Manufacturing
Headline: Dive into the World of Pharma Product Design and Commercial Manufacturing with Our Comprehensive Online Course! 🚀
Course Description:
Are you ready to unlock the secrets behind the scenes of pharmaceutical product development? From the initial design phase to the final stages of commercial manufacturing, our course "Pharma Product Design, Technology & Commercial Manufacturing" is your key to understanding the entire process as per the ICH Q8 guidelines. Led by the expert guidance of Hitendrakumar Shah, this comprehensive journey will equip you with the knowledge necessary to navigate the complexities of pharmaceutical product lifecycle management.
What You'll Learn:
-
Pharmaceutical Product Design 🛠️
- Understanding design space based on ICH Q8 guidelines
- Applying Quality by Design (QbD) principles to develop robust pharmaceutical products
-
Technology Transfer in Pharma 🔬
- Mastering the transfer of both analytical and manufacturing processes to a commercial setting
- Ensuring seamless integration of technology into production
-
Process Validation & Potent Molecules 🧪
- Learning about process validation requirements for your first commercial batches
- Special focus on the unique considerations when handling potent molecules with a dedicated lecture
-
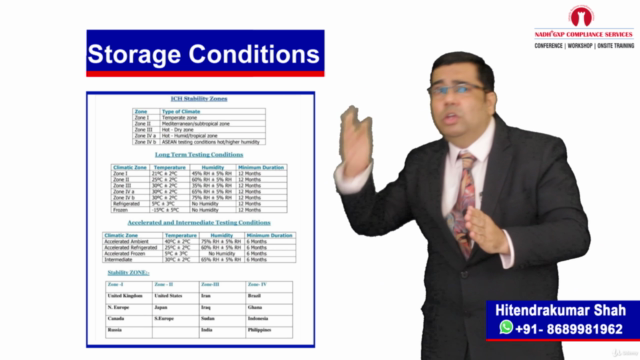
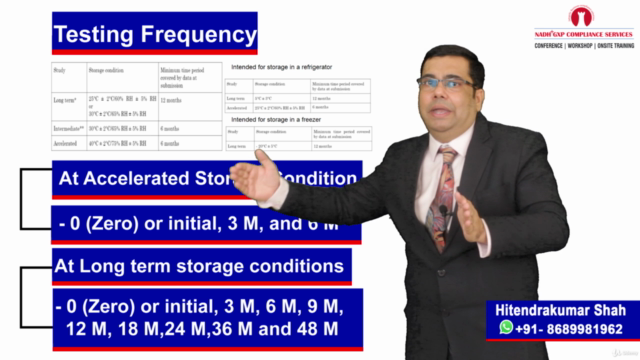
Stability Studies & Commercialization 📈
- Conducting in-depth stability studies post-validation to ensure product longevity and quality assurance
- Understanding the importance of yearly long-term stability studies to maintain product efficacy
Course Highlights:
-
Detailed Lectures: Each aspect of pharmaceutical product development is meticulously covered, from design to commercialization.
-
Expert Instruction: Benefit from the extensive knowledge and experience of Hitendrakumar Shah, a seasoned course instructor in the field.
-
Practical Application: Gain insights into real-world scenarios and learn how to apply these concepts to your own pharmaceutical projects.
-
Comprehensive Content: This course is packed with valuable information, including lectures on technology transfer and handling potent molecules, as well as the critical step of stability studies.
Who Should Take This Course? 👩🔬👨🏫
- Aspiring and current professionals in the pharmaceutical industry
- Researchers looking to transition into pharmaceutical development or manufacturing roles
- Regulatory affairs specialists
- Quality assurance personnel
- Any individual interested in the pharmaceutical product lifecycle from design through commercialization.
Embark on your journey to mastering the art and science of pharma product design, technology transfer, and commercial manufacturing. Enroll in our course today and take a step closer to becoming a leading expert in the field! 🎓💊
Course Gallery


Loading charts...