Organic Chemistry - Alcohols Phenols & Ethers

Why take this course?
🎓 Organic Chemistry - Alcohols, Phenols & Ethers: Complete Chemistry for Engg and Medical Entrance Exam Preparation (IIT JEE Main | Advanced | BITSAT | SAT | NEET, etc.)
Course Summary
Welcome to the comprehensive course on Organic Chemistry with a focus on Alcohols, Phenols, and Ethers – critical topics for aspiring engineers and medical professionals preparing for competitive exams like IIT JEE Main, Advanced, BITSAT, SAT, NEET, and more. This course is designed to demystify the complex world of organic compounds and prepare you with the deep understanding and problem-solving skills required to excel in these rigorous tests.
Key Concepts Covered
🔍 Classification of Compounds:
- Alcohols and phenols are classified based on the number of hydroxyl groups and the hybridisation of the carbon atom they're attached to (sp3 or sp2).
- Ethers are classified by the groups attached to the oxygen atom.
🚀 Preparation Methods:
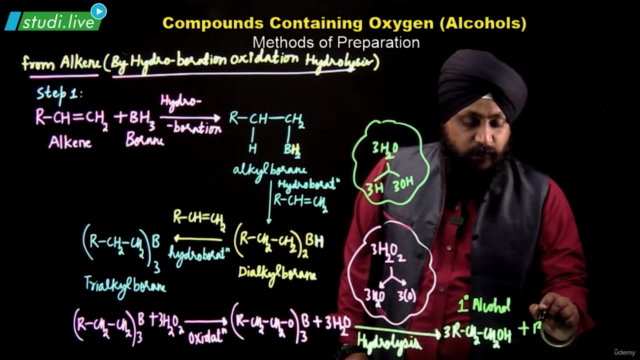
- Alcohols can be synthesized through hydration of alkenes, hydroboration-oxidation reaction, and from carbonyl compounds via catalytic reduction or Grignard reagents.
- Phenols are prepared by substituting halogen atoms or sulphonic acid groups with a –OH group, through hydrolysis of diazonium salts, and industrially from cumene.
🔋 Physical Properties:
- Alcohols have higher boiling points than comparable hydrocarbons, ethers, and haloalkanes due to their ability to form intermolecular hydrogen bonds with water.
🧬 Chemical Reactions:
- Alcohols undergo nucleophilic substitution with hydrogen halides, dehydration to form alkenes, and oxidation reactions that vary depending on the alcohol's carbon skeleton.
- Phenols exhibit acidic properties; electron withdrawing groups increase their acidity while electron releasing groups decrease it.
🔬 Phenol Reactivity:
- The presence of a –OH group in phenols activates the aromatic ring towards electrophilic substitution, particularly at ortho and para positions due to resonance effects.
- Reimer-Tiemann and Kolbe’s reactions are key phenol transformations, especially under alkaline conditions.
🎇 Ether Synthesis & Properties:
- Ethers can be prepared by dehydration of alcohols or through Williamson synthesis.
- They have boiling points similar to alkanes and solubility comparable to alcohols with the same molecular mass.
- The C–O bond in ethers is susceptible to cleavage by hydrogen halides.
- Alkoxy groups activate aromatic rings for electrophilic substitution, directing the incoming group to ortho and para positions.
Why This Course?
✅ Exam-Focused Content: Tailored specifically for the types of questions you'll encounter in top engineering and medical entrance exams.
📚 Detailed Explanations: Real-world applications and detailed explanations make complex concepts clear and easy to understand.
🧠 Interactive Learning: Engage with the material through interactive examples, practice problems, and quizzes to test your knowledge.
🤝 Expert Instructor: Learn from an experienced educator who specializes in teaching Organic Chemistry for competitive exams.
What You'll Achieve
🚀 Master Organic Chemistry: Gain a deep understanding of alcohols, phenols, and ethers, their reactions, and their significance.
📈 Improve Your Problem-Solving Skills: Learn to tackle challenging problems with confidence and accuracy.
🌍 Prepare for Success: Equip yourself with the knowledge needed to ace your upcoming entrance exams.
Enroll now and take the first step towards mastering Organic Chemistry – a fundamental subject area for success in your future career! 🎓✨
Course Gallery



Loading charts...