IVDR 2017 746 regulatory affairs: Learn EU compliance

Why take this course?
🎓 Master EU Compliance for In Vitro Diagnostic Medical Devices with IVDR 2017/746: A Step-by-Step Guide
🚀 Course Overview: This comprehensive online course, "IVDR 2017/746 Regulatory Affairs," is meticulously designed to simplify the complex EU regulations for Invitro Diagnostic Medical Devices (IVDDs). With clear, concise, and practical guidance, you'll gain a solid understanding of the requirements needed to achieve market approval in the European Union.
🔍 Course Structure: The course is structured into nine intuitive sections that systematically cover all aspects of IVDR compliance:
Section 1: Introduction to IVDR
- The introduction to IVDR 2017/746 and its significance.
- Understanding the shift from the old directive to the new regulation.
- Detailed transition timelines for a smooth move to IVDR.
Section 2: Economic Operators Explained
- A deep dive into the roles of European Authorised Representatives, Distributors, Importers, Manufacturers, and their obligations under IVDR.
- Clarifying when and how these responsibilities change.
- Emphasizing the importance of a person responsible for regulatory compliance.
Section 3: Understanding EUDAMED
- An introduction to EUDAMED, the EU Database for Medical Devices.
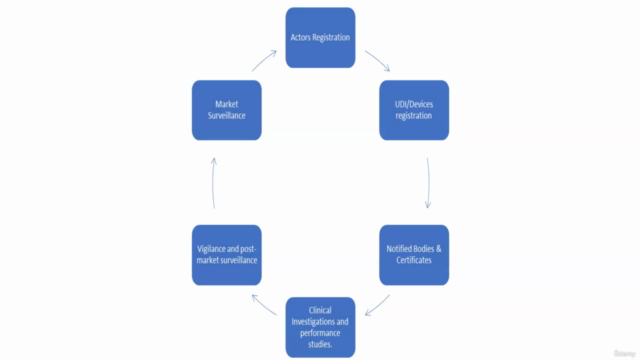
- A walkthrough on actor registration processes.
- Timelines for the transition and implementation of the EUDAMED system.
Section 4: Unique Device Identification (UDI) Registration
- Introduction to UDI and its necessity.
- Detailed explanation of the UDI carrier, including current accredited organisations.
- UDI timelines and how to attach it to devices and packaging.
Section 5: Classification of In Vitro Diagnostic Medical Devices
- Guidance on qualifying devices and determining if your product falls under IVDD.
- A clear understanding of IVD definitions, classification rules, and the impact of IVDR.
Section 6: Conformity Assessment Pathways
- A detailed guide to the conformity assessment routes for Class A to D devices.
Section 7: Sufficient Clinical Data & Safety Monitoring
- Insights into General Safety and Performance Requirements.
- Identifying the Intended Purpose of your device.
- Strategies for collecting sufficient clinical data and maintaining compliance through Clinical Evaluation and Post Market Performance Follow-up (PMPF).
Section 8: Post Market Surveillance
- A comprehensive look at post market surveillance requirements, including reporting and periodic safety updates.
- Understanding the role of Competent Authorities and the European Commission in market surveillance.
Section 9: Additional Topics & Considerations
- Discussing the Medical Device Coordination Group and its importance.
- Addressing Brexit and its implications for IVDD manufacturers.
- Exploring the Switzerland Mutual Recognition Agreement, Turkey and EFTA agreement with the EU.
- Understanding Common Standards and the Rolling Plan.
- The role of European Union Reference Laboratories in supporting IVDR implementation.
🌟 Course Benefits:
- Save time with a clear, step-by-step understanding of IVDR requirements.
- Gain a profound grasp of regulations in simple terms.
- Prioritize your work effectively to meet the IVDR 2017/746 timelines.
- Discover the reasons behind these regulations and their impact on your business.
- Learn about your specific obligations under the new regulation.
- Understand the EUDAMED system, including registration processes and timelines.
- Get practical examples of UDI carriers creation.
- Learn how to classify IVDDs correctly.
- Navigate the conformity assessment routes for different types of devices.
- Collect sufficient clinical data to ensure compliance.
- Master market surveillance requirements post device launch.
- Post-Brexit insights for both EU and UK manufacturers.
- Understand the roles and responsibilities regarding IVDR in Turkey and EFTA countries.
- Learn how European Union Reference Laboratories can support your compliance efforts.
🎉 Who Should Take This Course? This course is designed for:
- Medical Device Manufacturers (both large companies and startups)
- Quality Assurance and Regulatory Affairs Professionals
- R&D Teams
- Anyone involved in the manufacturing, import, or distribution of IVDDs who wish to ensure compliance with EU regulations.
📅 Enroll Now to Stay Ahead: Stay informed, compliant, and competitive in the fast-paced world of medical devices. Enroll in our "IVDR 2017/746 Regulatory Affairs" course today and secure your position as an industry leader. 🎓✨
Course Gallery



Loading charts...