ISO 13485:2016 - Design and Development of Medical Devices
Design and Development of Medical Devices in the perspective of ISO 13485:2016 and Medical Devices Industry
4.02 (438 reviews)

1 119
students
2 hours
content
Jun 2025
last update
$59.99
regular price
Why take this course?
🌟 Master ISO 13485:2016 - Design & Development of Medical Devices 🌟
Course Overview:
Embark on a comprehensive learning journey with our "ISO 13485:2016 - Design and Development of Medical Devices" course, specifically tailored for industry professionals and those looking to master the quality management system for medical devices as per the latest international standards.
Course Highlights:
- Premium Quality Learning: Dive into a high-quality educational experience that covers the nuances of ISO 13485:2016 in relation to regulatory requirements and industry best practices.
- Complex Process Simplified: The design and development process for medical devices can be intricate, but this course breaks it down into manageable, understandable segments.
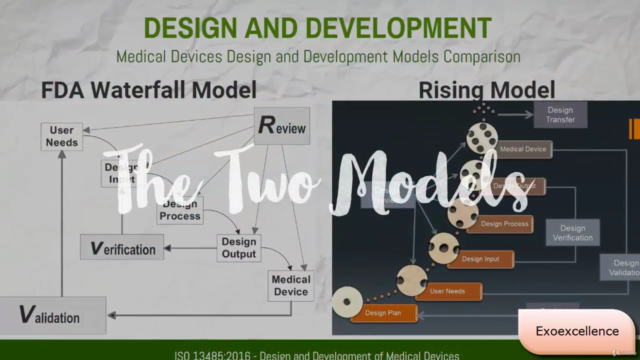
- Unique Learning Model: Benefit from the unique Rising Model developed by our expert instructor, Waqas Imam, which offers a fresh perspective on ISO 13485:2016 requirements.
What You Will Learn:
- Introduction to ISO 13485:2016
- FDA Waterfall Model for Design and Development
- Rising Model for Design and Development
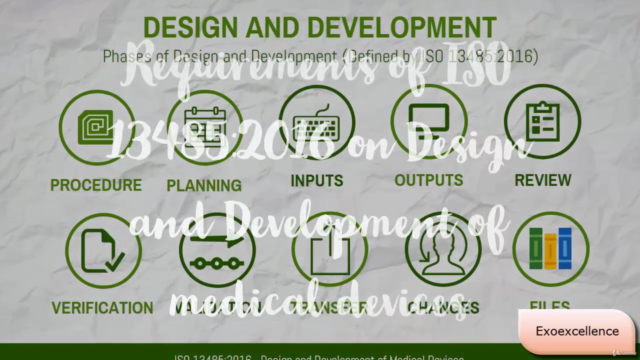
- Design and Development Planning
- Design and Development Procedure
- Design and Development Inputs and Outputs
- Design and Development Review, Verification & Validation
- Design and Development Transfer
- Design and Development Changes
- Design and Development Files Management
- ISO 13485 Requirements on Design and Development
Course Benefits:
- Economical Learning: Avoid expensive one-day workshops. This course provides a comprehensive understanding at a fraction of the cost.
- No ISO Standard Copy Issue: A copy of the standard is not included with this course due to copyright restrictions, but all content aligns with the standard.
Included Resources:
- Free PDF Slides Presentation
- Free PDF Rising Model of Design and Development
Additional Features:
- Practice Exam: Test your knowledge with a practice exam included in the course.
- Certificate of Completion: Earn a certificate upon successful completion of the course and passing the exam.
- One Month Money Back Guarantee: Register with confidence, knowing you can get a full refund within one month if not satisfied.
- Exoexcellence Training Resources Certificate: Opt for our exclusive certificate that addresses student concerns regarding Udemy's recent template changes.
- Support and Interaction: Use the discussion forum to ask questions, share insights, and report any issues with course content.
Enrollment Process:
Ready to begin your journey towards mastering ISO 13485:2016 for medical device design and development? Click the "Take This Course" button now, follow the instructions, and start your path to professional excellence in the medical devices industry!
Join us today and transform your understanding of ISO 13485:2016 and its application in design and development processes. 🎓
Course Gallery


Loading charts...
Related Topics
2203278
udemy ID
07/02/2019
course created date
08/02/2020
course indexed date
Bot
course submited by
