In-vitro Studies for Solid Oral Dosage Forms in Pharma
Why take this course?
🎓 Explore In-vitro Studies for Solid Oral Dosage Forms in Pharma!
Course Overview:
This comprehensive online course, In-vitro Studies for Solid Oral Dosage Forms in Pharmacette, is meticulously designed to delve into the critical aspects of dissolution analysis, a cornerstone in drug development and quality control. Aydan Ozdenc, an experienced instructor, will guide you through the intricate details and technical requirements of dissolution analysis for New Drug Application (NDA) and Abbreviated New Drug Application (ANDA) submissions.
Why Enroll in This Course?
🚀 In-vitro & In-vivo Studies: Understand the importance of both in-vitro dissolution data and in-vivo bioavailability or bioequivalence studies in characterizing the quality and performance of drug products.
🔬 Dissolution Testing: Learn how dissolution testing serves as a key control tool to ensure the consistency of product manufacturing and the bioperformance of solid oral dosage forms without the need for in-vivo performance.
Course Breakdown:
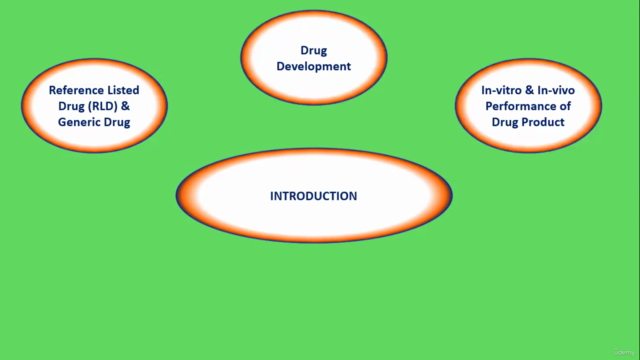
Module 1: Introduction Dive into the fundamentals of dissolution testing and its significance in the pharmaceutical industry.
Module 2: In-vitro & In-vivo Studies for Drug Product
- Reference Listed Drug (RLD) & Generic Drug
- Drug Development
- In-vitro & in-vivo Performance of Drug Product
Module 3: Solubility Explore the role of solubility in drug formulation and dissolution, including its impact on drug absorption.
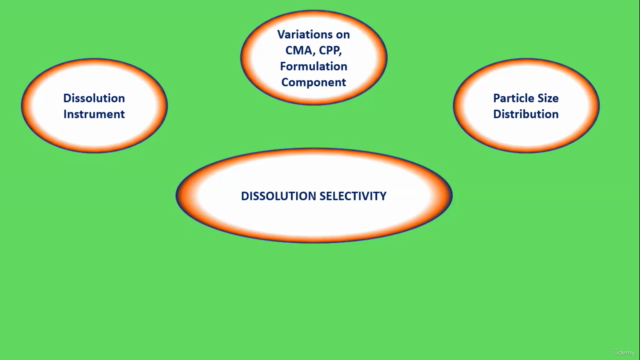
Module 4: Dissolution & Dissolution Selectivity
- Dissolution-1 (Dissolution Instrument)
- Dissolution-2 (Variations on CMA, CPP, Formulation Component & Dissolution Selectivity)
- Dissolution-3 (Particle Size Distribution & Dissolution Selectivity)
- Dissolution-4 (Polymorphic Structure & Dissolution Selectivity)
- Dissolution-5 (Excipients Composition & Dissolution Selectivity)
- Dissolution-6 (Critical Process Parameters & Dissolution Selectivity)
Module 5: Dissolution Test as a Tool Understand how dissolution methods can be utilized both in formulation development and as a quality control tool.
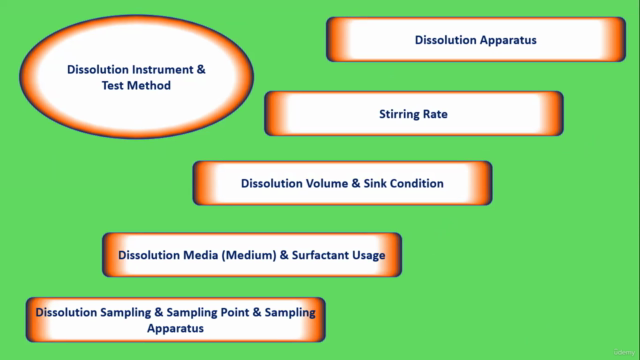
Module 6: Dissolution Test Method Get hands-on with the practical aspects of dissolution testing, including apparatus selection, media usage, and sampling procedures.
Module 7: Evaluation of Dissolution Test Results Learn how to interpret and evaluate the results of dissolution tests using tools like the Similarity Factor (f2).
Module 8: Selectivity of Dissolution Method Analyze the selectivity of dissolution methods and its importance in ensuring product quality.
Module 9: Dissolution in Pharmaceutical Development Discover how dissolution testing is integral to pharmaceutical development, including discriminative dissolution method development.
Module 10: Dissolution in ICH Examine the role of dissolution testing within the International Council for Harmonisation (ICH) guidelines, with a focus on immediate and extended release tablets.
Module 11: Conclusion Wrap up the course with a comprehensive understanding of the regulatory landscape and practical applications of in-vitro dissolution studies.
Learning Outcomes:
Upon completion of this course, you will have a robust grasp of:
- The significance of dissolution analysis in drug development and quality assurance
- How to conduct and interpret dissolution testing using the latest methodologies
- The role of dissolution testing in regulatory submissions and compliance with ICH guidelines
- Strategies for optimizing oral dosage formulations based on dissolution data
Join us to unlock the secrets of in-vitro dissolution studies and elevate your expertise in pharmaceutical science! 🌟
Course Gallery
Loading charts...