Mastering ICH GCP:Ethical Clinical Trial Conduct& Compliance

Why take this course?
Course Headline: 🚀 Unlocking ICH-GCP E6 R2: A Complete Guide to Good Clinical Practice in Clinical Research 🚀
Course Description:
Embark on a transformative journey through our comprehensive online course, "Mastering ICH-GCP E6 R2: Good Clinical Practice for Clinical Research." This meticulously crafted program is your gateway to becoming a proficient and certified expert in the intricacies of conducting ethical and high-quality clinical trials.*
Why Take This Course?
- Tailored Curriculum: Designed for both novices and seasoned professionals, this course delves into the International Conference on Harmonization (ICH) guidelines, specifically E6 R2.
- In-Depth Understanding: Gain a profound grasp of Good Clinical Practice (GCP) in clinical research through dynamic multimedia content, interactive modules, and real-world case studies.
- Safety & Ethics: Master the core principles of ICH-GCP E6 R2 to ensure participant safety, data integrity, and ethical conduct in clinical trials.
- Expert Guidance: Led by industry-renowned instructors, each a luminary in their respective fields, you'll explore critical topics and practical insights.
- Real-World Application: Learn about obtaining informed consent, protocol adherence, adverse event reporting, investigator responsibilities, and the role of Institutional Review Boards (IRBs).
- Monitoring & Compliance: Gain effective monitoring techniques to maintain compliance with ICH-GCP standards.
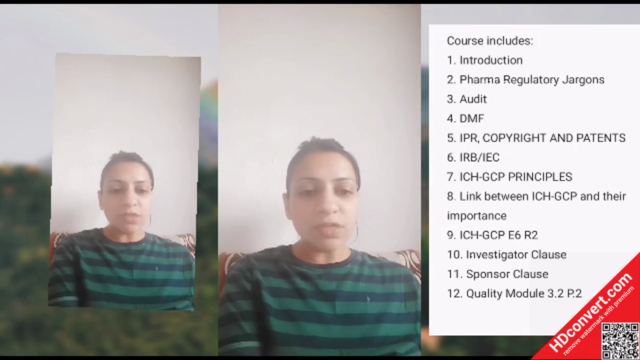
Course Outline:
- Introduction to ICH-GCP E6 R2: Overview of the guideline's purpose and structure.
- Fundamentals of GCP: The principles and practice of conducting clinical trials in accordance with GCP.
- Ethical Considerations: Understanding ethical implications and how they guide trial conduct.
- "Informed Consent" Deep Dive: Best practices for ensuring participants are fully informed and understand their rights and responsibilities.
- Trial Protocols & Design: How to design a study protocol that is compliant with ICH-G6 E6 R2.
- Data Management & Integrity: Ensuring the integrity of clinical data from collection to reporting.
- Pharmacovigilance & AE Reporting: Effective strategies for managing and reporting adverse events in trials.
- Regulatory Compliance: Understanding the role of regulatory bodies and compliance with international standards.
- Monitoring & Auditing: Techniques and best practices for monitoring, auditing, and quality assurance in clinical trials.
- Case Studies & Interactive Quizzes: Real-world scenarios to test your knowledge and understanding of GCP principles.
- Certification Exam: A comprehensive final exam to earn your certification in ICH-GCP E6 R2.
What's Included?
- Access to Course Materials: All the multimedia content, lectures, and materials you need to master ICH-GCP E6 R2.
- Expert Instruction & Support: Our industry-renowned instructors are there to guide you through each step of the course.
- Peer Interaction: Engage with fellow learners and share insights in our collaborative learning environment.
- Flexible Learning: Study at your own pace, from anywhere in the world, with 24/7 access to the course materials.
- Certification & Accreditation: Upon successful completion, earn a prestigious certification that will enhance your career in clinical research.
Who Should Enroll?
- Clinical Research Professionals
- Clinical Trial Coordinators and Managers
- Regulatory Affairs Specialists
- Healthcare Professionals Involved in Clinical Trials
- IRB/IEC Members & Personnel
- Students and Academics Interested in Clinical Research
Ready to Master ICH-GCP E6 R2? 👩🏫👨💼 Click "Enroll Now" to begin your journey towards excellence in Good Clinical Practice today!
Note: This course includes additional quizzes and an extra lecture specifically tailored for the 2023-24 academic year to ensure you have the most up-to-date knowledge of ICH-GCP E6 R2.
Course Gallery


Loading charts...