Good Laboratory Practices

Why take this course?
Course Headline: 🧬 Master Good Laboratory Practices with Expert Insights!
Course Title: Good Laboratory Practices
Instructor: Hitendrakumar Shah
Course Description:
Embark on a comprehensive journey into the world of Good Laboratory Practices (GLP) with our meticulously crafted online course. Good Laboratory Practices is designed to provide you with an in-depth understanding of the critical aspects that ensure data integrity, compliance with regulations, and overall quality control within analytical laboratories and microbiology labs.
Course Overview:
This course will cover a wide range of topics, from adhering to FDA/EU Citations to understanding the latest trends in data integrity as observed by inspectors. You'll explore the nuances of Data Integrity, with a focus on both Non-IT and IT aspects, including practical examples and current trends in inspector assessments.
Key Areas of Focus:
- Data Integrity in Analytical and Microbiology Laboratories: We'll delve into specific examples, such as Data Integrity in Microbiology laboratories, to give you a clear understanding of best practices and common pitfalls.
- Guidelines on Data Integrity: Learn about the various guidelines that govern data integrity, including FDA warning letters and EU GMP Annex 16.
- Understanding ALCOA+: Get to grips with the ALCOA+ principle, which is essential for maintaining the integrity of your laboratory data.
- Audit Trails and Sterility Tests: Understand when and how to repeat sterility tests and the importance of maintaining a robust audit trail in microbiology.
Diving Deeper into Analytical Laboratory Practices:
The course also covers essential aspects of analytical method validations, stability studies, and handling out-of-specification (OOS) or out-of-trend (OOT) investigations. You'll learn:
- Analytical Method Validation: From FDA expectations to the practicalities of QBD and QRM approaches, ICHQ14 for analytical method development, and the detailed parameters for ICHQ2(R1) validation.
- Validation Evaluation and Revalidation Criteria: Gain insights into how to evaluate methods and understand when revalidation is necessary.
Investigating Human Errors in Analytical Laboratories:
A dedicated lecture in this course will focus on the most common problems in analytical laboratories: human errors. You'll learn about different types of human errors, how to investigate them effectively, and strategies to avoid them. This knowledge is crucial for maintaining high standards of data integrity and ensuring the reliability of laboratory results.
Why Take This Course?
- Practical Application: Real-world examples and case studies will help you apply what you learn directly to your work environment.
- Regulatory Compliance: Stay updated with the latest FDA/EU citation trends and understand how they impact your laboratory practices.
- Data Integrity Mastery: From understanding the concept of ALCOA+ to mastering data integrity in microbiology, this course will equip you with the knowledge you need.
- Comprehensive Learning: Engage with a wealth of resources, including guidelines, validation parameters, and investigation techniques.
- Expert Led: Learn from an experienced professional, Hitendrakumar Shah, who brings years of industry expertise to your learning experience.
Enroll Now to Transform Your Laboratory Practices! 🔬✏️✨
Take the first step towards excellence in laboratory practices by enrolling in this insightful and practical online course today. Elevate your career and ensure the highest quality standards in your laboratory operations. Let's embark on this transformative learning journey together!
Course Gallery


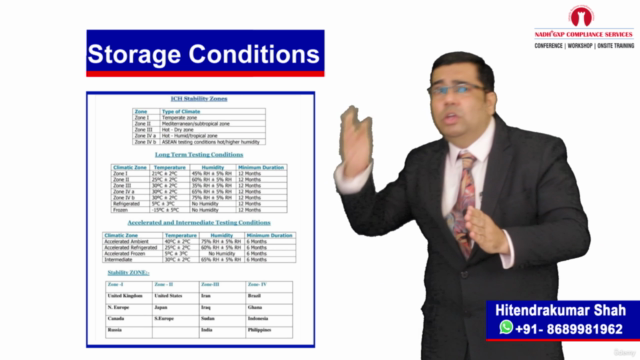
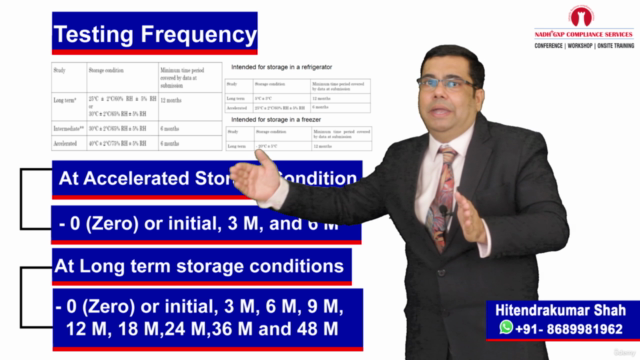
Loading charts...