Business intelligence for bio/pharma drugs - DrugPatentWatch

Why take this course?
🚀 Your Next Billion Dollar Blockbuster: Business Intelligence for Bio/Pharma Drugs 🌐
Course Overview:
Get insights into the biggest revenue-changing events in the pharmaceutical industry by mastering the art of tracking patent expirations, patent litigation, generic and biosimilar development. This course, Business Intelligence for Bio/Pharma Drugs, is designed to help you anticipate market-shaping forces and stay one step ahead in a competitive landscape.
Key Learnings:
- Patent Expiration Tracking: 🗓️ Understand when key drug patents expire to prepare for generic competition.
- Stronger Patents: ✍️ Learn how to draft patents that withstand legal scrutiny and protect your intellectual property.
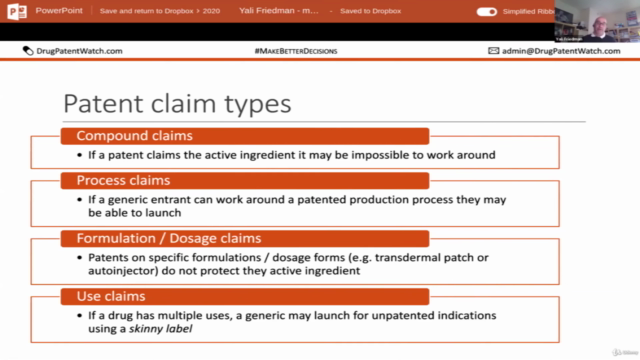
- Challenging Drug Patents: 🔍 Discover strategies for defeating drug patents and navigating complex legal challenges.
- Generic Market Entry: 💊 Evaluate opportunities for generic market entry and develop actionable plans to capitalize on these openings.
- Proprietary Deals Analysis: 🤝 Gain insights into out-of-court settlements and deal terms that can influence your strategic decisions.
Course Highlights:
- Comprehensive Coverage: 📊 Dive into commercial, legal, and regulatory factors affecting drug development and delivery.
- Real-World Examples: 🌟 Analyze case studies from the industry to apply your knowledge in practical scenarios.
- Strategic & Tactical Planning: 🚀 Develop a solid foundation for strategic thinking with tactical plans that can adapt to future market changes.
Included with Your Enrollment:
- FREE Companion Textbook: 📖 Receive a textbook that complements the course material, adding depth to your understanding of drug business intelligence.
Course Development Background:
This course is the culmination of over two decades of expertise from Yali Friedman, Ph.D., an industry veteran and recognized authority in the field. Dr. Friedman's pioneering work includes developing one of the first websites on the business of biotechnology in the 1990s and editing the Journal of Commercial Biotechnology. His extensive experience with DrugPatentWatch, a leading platform for tracking drug patents, has equipped him with the knowledge to guide you through the intricate world of pharmaceutical business intelligence.
Why Take This Course?
- Actionable Intelligence: 🎯 Gain insights that translate into actionable strategies for your role within the drug development and delivery value chain.
- Up-to-Date Knowledge: ⏳ Navigate through the ever-evolving legal and regulatory landscape with confidence, as this course is updated to reflect current trends and practices.
- Strategic Relevance: 🔍 Whether you're from a generic or branded company, this course provides valuable strategic insights that are applicable across the pharmaceutical industry spectrum.
- Comprehensive Understanding: 📊 Aim to fill knowledge gaps and deepen your understanding of commercial dynamics in pharmaceutical and biotechnology drugs.
- Additional Resources: 🔗 Explore additional books and web-based resources for deeper technical dives when needed.
About the Author:
Yali Friedman, Ph.D., is a renowned expert in business intelligence for life science companies, with over 20 years of experience. He has been recognized as one of the 100 most influential people in biotechnology by Scientific American. His contributions extend to authoring an MBA-level textbook, "Building Biotechnology," and publishing the Journal of Commercial Biotechnology. With a career dedicated to illuminating the intersection of science and business, Dr. Friedman's expertise is unparalleled in guiding strategic decisions in the competitive landscape of bio/pharma drugs.
Embark on this journey to discover how to anticipate market shifts, capitalize on opportunities, and navigate the complexities of the pharmaceutical industry with Business Intelligence for Bio/Pharma Drugs. Enroll now and unlock the potential of your next billion-dollar blockbuster! 💊💫🚀
Course Gallery



Loading charts...