(DOE) Design of Experiement in Pharmaceutical Development

Why take this course?
🌟 Course Title: (DOE) Design of Experiment in Pharmaceutical Development 🚀
Headline: Master the Art of Pharmaceutical Formulation with Cutting-Edge Design of Experiment Techniques! 🧫✨
Overview: Dive into the world of Design of Experiments (DoE) for Pharmaceutical Development and transform your approach to formulation design and process optimization. This comprehensive course is tailored for professionals eager to embrace the scientific method to its fullest potential, ensuring robust and quality outcomes in pharmaceutical development.
Why Enroll? 🤔
- Cost-Effective: Affordable access to high-quality education without compromising on content.
- Risk Assessment & Critical Parameter Identification: Gain insights into the importance of identifying critical parameters and assessing risks associated with formulation variations.
- Hands-On Learning: Engage with real-world case studies and practical exercises that bring theory to life.
- Latest Techniques & Tools: Learn the most advanced DoE techniques, including Two-Level Factorial Designs, Fractional Factorial Designs, Response Surface Methodology, and more.
- Project-Based Approach: Collaborate with peers in project teams to apply DoE principles to actual pharmaceutical development challenges.
Course Highlights:
- Introduction to DOE: Understand the fundamentals of Design of Experiment, its definitions, and the importance of systematic experimentation.
- Guide to Experimentation: Learn how to plan, implement, and analyze experiments effectively, with a focus on case studies for practical application. 3.🎯 Two-Level Factorial Designs: Master the design matrix, calculation matrices, and interpret main effects and interactions, with a deep dive into center points.
- Identifying Significant Effects: Discover how to identify significant location effects, determine statistical significance, and analyze replicated and non-replicated designs.
- Developing Mathematical Models: Learn the process of developing first-order models, residuals analysis, and model validation for optimal response optimization.
- Fractional Factorial Designs: Explore the structure of fractional factorials, identify optimal fractions, understand confounding/aliasing, and more.
- Proportion & Variance Responses: Tackle complex responses with sample size determination for proportion responses, identifying significant proportion effects, and handling variance responses.
- Response Surface Methodology: Delve into Central Composite Designs and Box-Behnken Designs to optimize multiple characteristics simultaneously.
- DOE Project Work: Collaborate in project teams to plan, conduct, analyze, and strategize next steps for real DoE projects in pharmaceutical development.
Who Should Attend? 👩🏫👨🔬
- Research Scientists
- Process Engineers
- Quality Assurance Professionals
- R&D Personnel
- Graduate Students in Pharmaceutical Sciences
- Anyone involved in the development or optimization of pharmaceutical processes and formulations
Key Takeaways:
- A comprehensive understanding of DoE principles and their application in the pharmaceutical industry.
- Skills to design and conduct experiments that lead to high-quality, reliable results.
- Techniques for data analysis and interpretation that will enhance decision-making processes in formulation development.
- Knowledge of advanced statistical tools and methods used in DoE to optimize pharmaceutical products and processes.
📆 Upcoming Start Dates: [Insert Dates Here] 🤝 Join Us & Elevate Your Expertise in Pharmaceutical Formulation Development! 🚀
Don't miss this opportunity to sharpen your skills and stay ahead in the rapidly evolving field of pharmaceutical development with Design of Experiment. Sign up today and see you in the class! 📚➡️🎉
Course Gallery
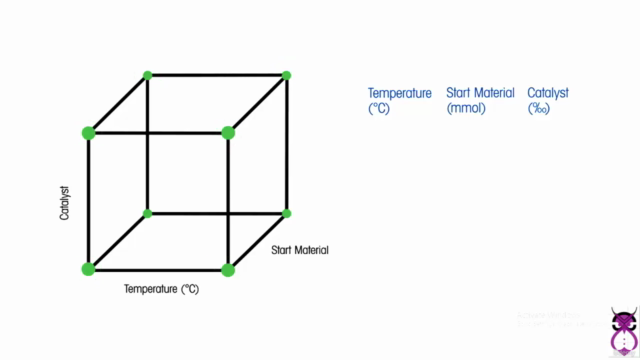
Loading charts...