CTD NeeS & eCTD compilation and submission of the dossiers

Why take this course?
🎓 Course Title: Mastering CTD NeeS & eCTD Compilation and Submission for Pharmaceutical Dossiers
Journey to CTD NeeS eCTD Submissions
Unlock the Secrets of Global Regulatory Compliance! 🌟
Course Description:
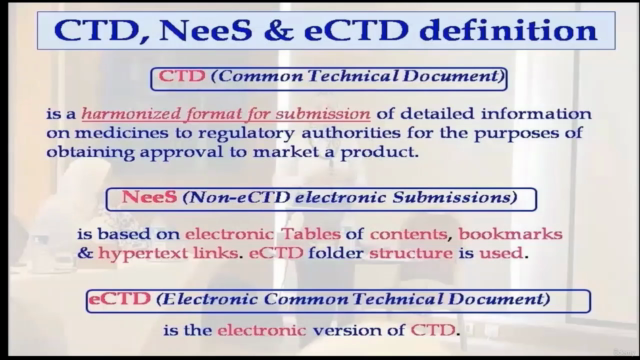
Understanding the Global Language of Pharmaceutical Registration CTD ("Common Technical Document") is the universal language for regulatory submissions across the globe. This comprehensive course delves into the rationale behind CTD, NeeS (& eCTD), providing meticulous guidance on their structure and format, which are crucial for pharmaceutical companies aiming to navigate the complex landscape of drug registration in different regions.
As pharmaceutical markets expand globally, it's imperative for companies to adapt to the diverse regulatory requirements. The MENA region and many global authorities have embraced eCTD/NeeS or CTD formats as the standard for submitting pharmaceutical products. This course is your key to understanding these systems and preparing your company for successful international registrations.
Why Enroll in this Course?
- Global Compliance: Learn how to prepare submissions that meet the requirements of regulatory authorities worldwide.
- Market Expansion: Gain insights into the global unified registration system, CTD, which is essential for exporting pharmaceutical products and attracting foreign investments.
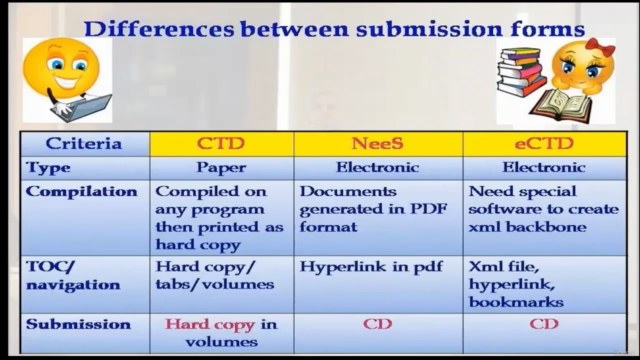
- Regulatory Mastery: Acquire the knowledge to understand the nuances between CTD, NeeS, and eCTD, ensuring your company's documentation stands out.
Course Outline:
- The Role of Regulatory Affairs: An overview of the critical function regulatory affairs play in drug development and approval processes.
- Decoding CTD, NeeS & eCTD: Learn the differences between these three systems and how they impact your pharmaceutical dossier.
- A Historical Perspective: Trace the evolution of CTD/eCTD and its global adoption.
- Organization of CTD: Dive into the detailed structure of a CTD document, including modules 1 to 5.
- Module 1: Administrative Information
- Module 2: Periodic Progress Reports (if applicable)
- Module 3: Clinical Study Reports
- Module 4: Data Management and Statistical Methodology (if applicable)
- Module 5: Nonclinical and Clinical Summaries
- Dossier Preparation Criteria: Understand the criteria for preparing a compliant dossier that meets international standards.
Who Should Attend? This course is designed for professionals in the pharmaceutical industry, including but not limited to:
- Regulatory Affairs Managers and Specialists
- Quality Assurance and Control Personnel
- Clinical Research Associates
- Medical Writers
- Drug Development Professionals
By completing this course, you'll be well-equipped to navigate the intricacies of CTD, NeeS, and eCTD submissions. Take the first step towards ensuring your pharmaceutical products are ready for international markets and comply with global regulatory standards! 🚀
Enroll now and embark on a transformative journey into the world of global pharmaceutical submissions with our expert-led course on CTD NeeS & eCTD Compilation and Submission. 🎓✨
Course Gallery


Loading charts...