Computerised System Validation (CSV) as per GAMP5 Guideline

Why take this course?
🎓 Course Title: Computerised System Validation (CSV) as per GAMP5 Guideline
Course Headline: Master CSV with cGXP Assessment, QRM of Computerised Systems, Vendor Evaluation, and Your Validation Master Plan!
Unlock the Full Scope of Computerised System Validation 💻
Many professionals approach computerized system validation as a mere checklist of IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification). However, true validation encompasses so much more. This comprehensive course will guide you through the intricacies of computerised system validation according to GAMP5 guidelines, ensuring you understand the depth and breadth of this critical process.
Course Structure:
This course is meticulously structured into 6 sections and 7 lectures, designed to provide a thorough understanding of each aspect of CSV. Throughout the course, feel free to ask questions in the comments section, where I, your instructor Hitendrakumar Shah, will be happy to clarify any doubts you may have.
-
Common Errors Related to Computerised Systems 🚫
- Understand typical mistakes made during validation and how to avoid them.
-
cGXP Assessment of Computerised Systems 🎯
- Learn the importance of assessing cGXP impact before diving into validation studies.
- Grasp regulatory expectations and understand FDA citations.
- Discover the 11 keys for effective cGXP Assessment.
- Identify common errors and take key lessons from the session.
- Engage in a Q&A to clear up any confusion.
-
Vendor Evaluation of cGXP Computerised Systems 🔍
- Comprehend the necessity of evaluating vendors for cGXP compliance.
- Learn about guideline requirements from EU, FDA, and GAMP.
- Dive into risk-based decision making and postal vs. onsite assessment methods.
- Participate in a Q&A to address your queries.
-
Validation Master Plan (VMP) and Quality Risk Management of cGXP Computerised Systems 📊
- Learn how to prepare a VMP that aligns with guidelines from EU and FDA.
- Understand the 7 key elements of VMP and their detailed discussions.
- Explore common errors in VMP preparation.
- Engage with the lecture on Quality Risk Management, covering its purpose, scope, and practical approaches.
- End with a Q&A to ensure you fully grasp the concepts.
-
Preparing Your Organisation for Inspections 🤝
- Ensure your organisation is inspection-ready by understanding the requirements and best practices.
-
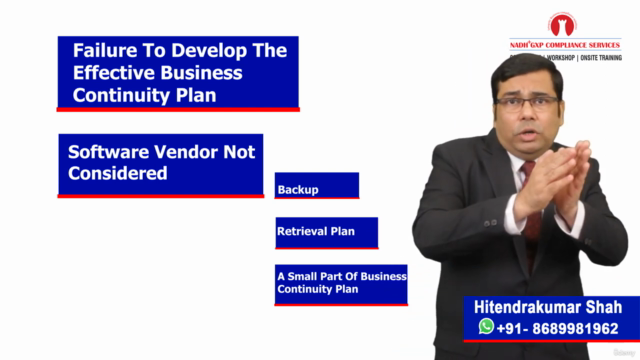
Business Continuity and Disaster Recovery Plan 🛡️
- Learn why having a robust BCP/DRP is crucial for your computerised system validation.
By completing this course, you will be well-equipped to manage the complexities of computerized system validation in compliance with GAMP5 guidelines. Whether you're involved in cGXP, QRM, vendor evaluation, or creating a VMP, this course will provide you with the necessary knowledge and skills to excel in your role.
Enroll now to embark on your journey to becoming a CSV expert! 🎫
Don't miss out on this opportunity to elevate your expertise in computerised system validation. Join us and transform the way you approach CSV today!
Course Gallery


Loading charts...