Clinical Trial Foundations

Why take this course?
🧑⚕️ Clinical Trial Foundations [2024] 📚
Course Headline: 🚀 Introduction to Trial Design & Management with Case Studies
Course Description:
Embark on a journey through the intricate world of clinical trials with our comprehensive course, designed to demystify the process of trial design and management. 🛠️ With simplified explanations, clear diagrams, and practical use cases, Deepika Khedekar will guide you through the complexities of clinical research step by step. Whether you're a healthcare professional, a student, or an aspiring clinical researcher, this course will equip you with the knowledge to navigate the ever-evolving landscape of clinical trials. 🌱
What You Will Learn:
-
Basics of Clinical Trials: Get acquainted with the fundamental concepts and terminologies used in clinical research.
-
Designing a Clinical Trial: Understand the step-by-step process involved in designing a clinical trial, including selecting the appropriate study design, population, interventions, and outcomes.
-
Conducting a Clinical Trial: Gain insights into the execution phase of a clinical trial, from participant recruitment to data collection.
-
Analyzing Data & Interpreting Outcomes: Learn how to analyze clinical trial data effectively and draw meaningful conclusions to inform medical practice and policy.
-
Challenges in Clinical Trials: Explore the common challenges faced during the trial process and the strategies to overcome them.
-
Real-World Case Studies: Dive into three detailed case studies from industry leaders like Kintor Pharma, AstraZeneca, and Eli Lilly, offering a glimpse into real-world applications of clinical trial design and management.
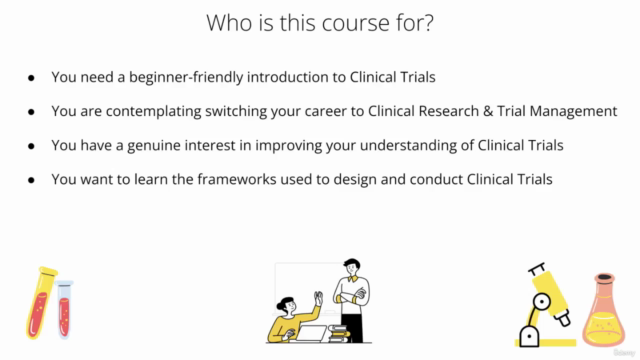
Who This Course Is For:
✅ You're interested in learning the basics of Clinical Trial Management.
✅ You're considering a career switch to Clinical Research & Management.
✅ You have a keen interest in understanding more about Clinical Trials.
✅ You want to grasp the standardized frameworks for designing and conducting clinical trials.
Why This Course?
-
Accessible Learning: This course is crafted to be accessible globally, ensuring that knowledge about Clinical Trial Foundations isn't just confined to a few.
-
Up-to-Date Content: As breakthroughs occur in clinical research, the course content will evolve to reflect the latest industry practices and methodologies.
-
Practical Approach: With a focus on practical applications, you'll be able to apply the concepts learned directly to real-world scenarios.
Join us on this enlightening journey into the world of clinical trials. Whether you're a seasoned professional or just starting out in the field, this course will provide you with a solid foundation in Clinical Trial Foundations. We look forward to having you aboard and hope you'll find as much joy and enlightenment in these studies as we have in creating this course. 🎓✨
Course Gallery

Loading charts...