Clinical Research- Advance Course (Audio+Test+Certificate)

Why take this course?
🏫 Clinical Research - Advanced Course (Audio+Test+Certificate) 🚀 Headline: Master the Ins and Outs of Clinical Trials, Regulatory Bodies, and Essential Documents!
Course Overview:
Course Objective:
Dive deep into the world of clinical research with our advanced course designed to elevate your understanding and skill set. This comprehensive program is tailored for students, Investigators, Study Coordinators, Sponsors, Clinical Project Managers, Monitors, and any other members of a clinical study team aiming to secure top-end positions in the clinical research industry.
Course Structure:
This advanced course is meticulously developed to align with industry expectations. It includes practical tests and case study-based scenarios to reinforce your learning and ensure you are well-prepared for real-world challenges. The curriculum is structured into 10 modules, each focusing on a specific aspect of clinical research:
Key Modules:
- Module 1: Understanding the Importance & Types of Clinical Trials
- Module 2: Clinical Trial Phases & Key Players at Glance
- Module 3: ICH GCP Principles
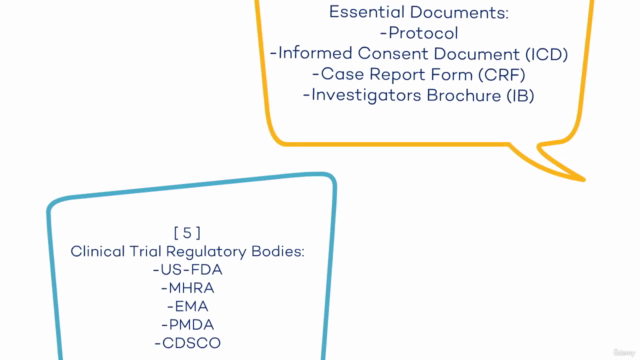
- Module 4: Essential Documents (Protocol, Informed Consent Document, Case Report Form, Investigator’s Brochure)
- Module 5: Clinical Trial Regulatory Bodies (US-FDA, MHRA, EMA, PMDA, CDSCO)
- Module 6: Audit & Inspection Essentials
- Module 7: Special Population Clinical Trials (Geriatric, Paediatric, and More)
- Module 8: Advanced Clinical Trial Designs
- Module 9: Real-World Case Studies
- Module 10: Clinical Trial Terminologies Decoded
Who Should Enroll?
This course is an ideal fit for:
- Graduate or postgraduate degree holders in Pharmacy
- Medical graduates or healthcare stream professionals
- Individuals with a background in Life Sciences
- Professionals from the healthcare industry
Learning Outcomes:
By completing this course, you will:
- Gain a comprehensive understanding of clinical research principles and processes
- Learn about the design and types of clinical trials that underpin new treatments and interventions
- Become familiar with essential documents in clinical research, including how to draft and manage them
- Understand the roles and responsibilities of different stakeholders in clinical trials
- Familiarize yourself with the regulatory landscape of clinical trials across various regions
- Learn about audit and inspection procedures within the clinical trial environment
- Explore the complexities of conducting clinical trials in special populations
- Enhance your knowledge of clinical trial terminologies to improve communication and understanding within the industry
Course Highlights:
- Expert-led audio lessons for a more interactive learning experience
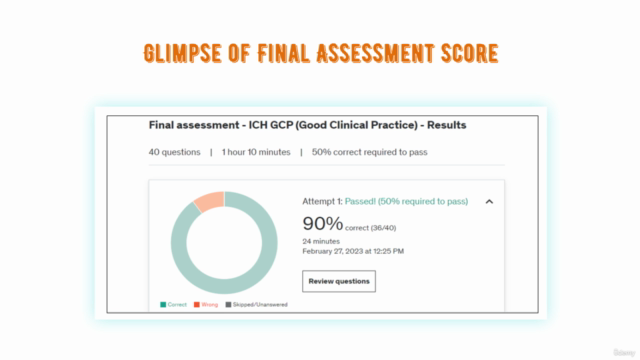
- Comprehensive practice tests to assess your understanding and knowledge retention
- A certificate of completion to showcase your advanced knowledge in clinical research
- Access to case study scenarios that reflect real-life clinical trials challenges
🎓 Embark on your journey to becoming a clinical research expert with ProRelix Education's Advanced Course – where learning meets practice, and expertise is proven. Enroll now and take the first step towards a successful career in clinical research!
Course Gallery


Loading charts...