Good Clinical Practice ICH GCP - Full Certificate Course

Why take this course?
🚨 Attention Aspiring Clinical Research Professionals! 🚨
🚀 Title: Certificate Course in ICH-GCP [Good Clinical Practice]
Are you passionate about clinical research and eager to ensure ethical and compliant studies? Look no further! Our Certificate Course in ICH-GCP [Good Clinical Practice] is the perfect opportunity for you to delve into the world of global clinical trial standards. 🔬🌍
Key Highlights of the Course:
-
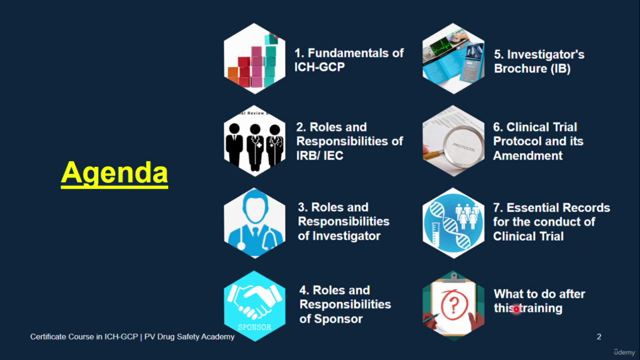
Essential ICH-GCP Principles: 🎓 Master the core principles of ICH-GCP, which are pivotal for ensuring ethical and scientific integrity in clinical trials. You'll understand the global acceptance of these guidelines, making your knowledge universally applicable.
-
Roles and Responsibilities of IRB/IEC: 🧐 Learn about the essential functions of Institutional Review Boards (IRBs) or Ethics Committees (IECs), including their composition, operations, and how they ensure ethical oversight.
-
Roles and Responsibilities of Investigator: 👩🔬 Understand the critical role of an investigator in clinical trials, from protocol compliance to informed consent, and everything in between – including managing investigational products and patient safety.
-
Roles and Responsibilities of Sponsor: 🏢 Grasp the oversight responsibilities of a trial sponsor, including their role in communication, quality management, and ensuring ethical conduct throughout the clinical trial process.
-
Investigator's Brochure: 📚 Discover how to develop an Investigator’s Brochure, which provides essential safety information about investigational products to all participants of a clinical trial.
-
Clinical Trial Protocol and its Amendment: 📝 Learn the ins and outs of clinical trial protocols, what they contain, and how they are amended – crucial for maintaining the integrity of research data.
-
Essential Records for the conduct of Clinical Trial: 📁 Manage essential records effectively to ensure the continuity and transparency of clinical trials.
Why Choose This Course?
-
Industry-Recognized Certification: 🏆 Set yourself apart with a certificate that is recognized by industry leaders, demonstrating your expertise in ICH-GCP.
-
Career Advancement: 🚀 Elevate your career in clinical research. With the knowledge you'll gain, you're well-equipped for roles in clinical trial management, monitoring, and regulatory affairs.
-
Global Opportunities: 🌎 ICH-GCP is a standard accepted worldwide. This course opens doors to international career opportunities, allowing you to contribute to global health advancements.
Who Should Enroll?
This course is ideal for:
- Clinical Research Associates (CRAs)
- Clinical Trial Coordinators
- Investigators
- Regulatory Affairs Professionals
- Anyone with an interest in ethical clinical research
📚 No prior knowledge of clinical research is required. However, a basic understanding of medical terminology is recommended.
Ready to Become a Prodigious Clinical Research Professional? 🚀
Enroll in our Certificate Course in ICH-GCP [Good Clinical Practice] today and take the first step towards an exciting and rewarding career in clinical research! 🌟
Enroll now and be part of a community committed to upholding the highest ethical standards in clinical research. Let's make a difference together!
Course Gallery

Loading charts...