Bioavailability (BA) / Bioequivalence (BE) in Pharma
Why take this course?
🎉 Explore in-vivo studies and learn bioavailability (BA)/bioequivalence (BE) context for pharmaceutical drug development! 🌊 🧐 Understanding Bioavailability & Bioequivalence
Bioavailability (BA) is a crucial aspect in pharmacology, indicating the extent and rate at which the active ingredient of a medication is absorbed into a patient's bloodstream and becomes available for use by the body. It's not just about how much of the drug reaches the blood—it's also about how quickly it happens.
Bioavailability Studies: These studies are essential in determining the fraction of the medication that enters systemic circulation after administration, either by injection or oral ingestion. They help in understanding the pharmacokinetics of a drug and its impact on efficacy and safety.
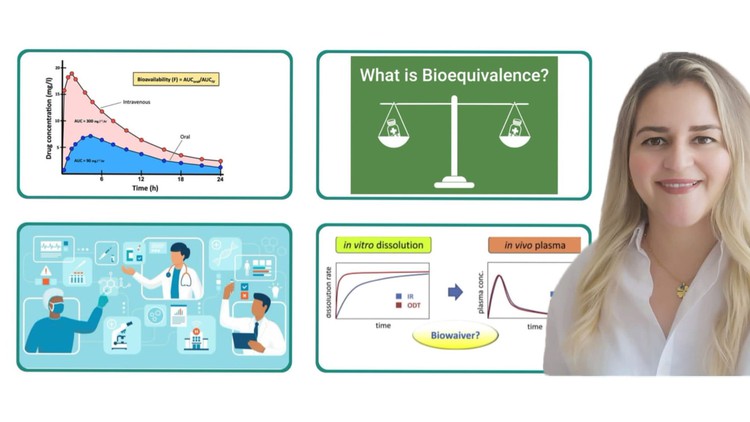
🔬 Diving into Bioequivalence (BE)
Bioequivalence assesses whether two medicinal products containing the same therapeutic ingredient, and whose quality is comparable, can be expected to have the same effect on the body under the conditions specified for the comparison.
Bioequivalency Studies: These studies are designed to compare the bioavailability of different formulations or brands of a medication to determine if they are equivalent in terms of rate and extent of absorption. The results of these studies support regulatory decision-making on product approval, interchangeability, and substitution.
🔥 Key Elements of BA & BE
- ADME: Absorption, Distribution, Metabolism, and Excretion are the steps to understand the pharmacokinetic path of a drug.
- Pharmacokinetics (PK): This is the study that describes the fate of an exogenous substance administered to a living organism. The main PK parameters include AUC (Area Under the Curve), Cmax (Maximum Concentration), and Tmax (Time to reach Cmax).
- Bioequivalency Criteria: These are statistical criteria that define how close two products must be in terms of their bioavailability for them to be considered bioequivalent.
📚 Study Designs
The course will cover different types of study designs used in BA/BE studies, such as:
- Cross-over Study Design: Subjects serve as their own control by receiving two treatments at different times.
- Parallel Study Design: Subjects are randomized to receive one treatment or the other, and there is an independent control group for each treatment.
👥 Study Population & Conditions
The selection of subjects, the number of subjects, and the health conditions of these subjects play a significant role in ensuring the validity of the study results.
📅 Sampling Times are critical for accurately measuring the drug's absorption profile.
🌍 Biowaiver Criteria
Biowaivers can be requested by sponsors to the regulatory agencies to waive certain pre-market approval requirements, based on demonstrated bioequivalence or biodegradation similarity between products.
📈 Biowaiver Criteria vary depending on the dosage form and the administration route, such as for orodispersible tablets, oral solutions, parenteral solutions, and emulsions.
🚀 Conclusion
By mastering the principles of bioavailability and bioequivalence, you'll gain a deeper understanding of how drugs are developed and approved, ensuring they are safe and effective for patients. This knowledge is invaluable for those working in pharmaceutical development, regulatory affairs, or clinical research.
Enroll now to embark on this journey into the world of BA/BE studies and become an expert in drug development and approval processes! 🎓🚀
Loading charts...